
A piston–cylinder device initially contains 1.2 kg of air at 700 kPa and 200°C. At this state, the piston is touching on a pair of stops. The mass of the piston is such that 600-kPa pressure is required to move it. A valve at the bottom of the tank is opened, and air is withdrawn from the cylinder. The valve is closed when the volume of the cylinder decreases to 80 percent of the initial volume. If it is estimated that 40 kJ of heat is lost from the cylinder, determine (a) the final temperature of the air in the cylinder, (b) the amount of mass that has escaped from the cylinder, and (c) the work done. Use constant specific heats at the average temperature.
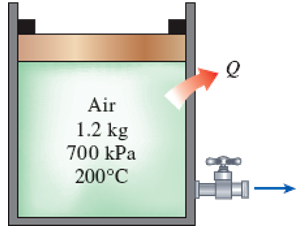
FIGURE P5–183
(a)
The final temperature of air in the cylinder.
Answer to Problem 185RP
The final temperature of air in the cylinder is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the piston-cylinder as the control volume. Initially the cylinder is filled with air and the valve is in closed position, further no other mass is allowed to enter the cylinder. Hence, the inlet mass is neglected i.e.
Rewrite the Equation (I) as follows.
Write the formula for initial volume of air present in the cylinder.
Here, the mass of air is
Write the formula for mass of air present in the cylinder at final state.
Here, the subscript 2 indicates the final state.
Write the energy balance equation.
Here, the heat transfer is
The pressure of
The Equation (V) reduced as follows.
Write the formula for boundary work done on the cylinder.
Here, the pressure required to move the piston is
The enthalpy and internal energy in terms of temperature and specific heats are expressed as follows.
Rewrite the Equation (VI) as follows.
The temperature of the air while exiting the cylinder is considered as the average temperature of initial and final temperatures.
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant
Refer Table A-2b, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
Substitute
It is given that the final volume is 80 % of initial volume.
Substitute
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (X) and obtain the value of
Thus, the final temperature of air in the cylinder is
(b)
The amount of mass escaped from the cylinder.
Answer to Problem 185RP
The amount of mass escaped from the cylinder is
Explanation of Solution
The amount of mass escaped from the cylinder is nothing but the mass of air vented out until final state i.e.
Refer Equation (II) and (IX).
Conclusion:
Substitute
Thus, the amount of mass escaped from the cylinder is
(c)
The work done.
Answer to Problem 185RP
The amount of mass escaped from the cylinder is
Explanation of Solution
The work done is nothing but the work done on the piston to move it i.e. boundary work
Refer part (a).
Thus, the work done is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- A 5-ft3 rigid tank initially contains refrigerant-134a at 60 psia and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 140 psia and 80F. The valve is now opened, allowing the refrigerant to enter the tank, and is closed when it is observed that the tank contains only saturated liquid at 100 psia. Determine (a) the mass of the refrigerant that entered the tank, (b) the amount of heat transfer with the surroundings at 708F, and (c) the entropy generated during this process.arrow_forwardNitrogen gas is compressed from 80 kPa and 27°C to 480 kPa by a 10-kW compressor. Determine the mass flow rate of nitrogen through the compressor, assuming the compression process to be polytropic with n = 1.3.arrow_forwardA rigid tank contains 5 kg of refrigerant-134a initially at 20°C and 140 kPa. The refrigerant is now cooled while being stirred until its pressure drops to 100 kPa. Determine the entropy change of the refrigerant during this processarrow_forward
- A rigid vessel contains 5.0 kg of wet steam at 0.4 MPa. After the addition of 9585 kJ the steam has a pressure of 2.0 MPa and a temperature of 700°C. Determine the initial internal energy and the specific volume of the steam.arrow_forwardA balloon initially contains 65 m3 of helium gas at atmospheric conditions of 100kPa and 22°C. The balloon is connected by a valve to a large reservoir that supplies helium gas at 150 kPa and 25°C. Now the valve is opened, and helium is allowed to enter the balloon until pressure equilibrium with the helium at the supply line is reached. The material of the balloon is such that its volume increases linearly with pressure. If no heat transfer takes place during this process, determine the final temperature in the balloon?arrow_forwardLiquid water at 200 kPa and 20 degress celcius is heated in a chamber by mixing it with superheated steam at 200 kPa and 250 degrees celcius. Liquid water enters the mixing chamber at a rate of 4.2 kg/s, and the chamber is estimated to lose heat to the surrounding air at 23 degress celcius at a rate of 1200 kJ/min. If the mixture leaves the mixing chamber at 200 kPa and 85 degrees celcius determine (a) the mass flow rate of the superheated steam, and (b) the rate of entropy generation during the mixing process.arrow_forward
- A 0.05-m3 rigid tank initially contains refrigerant134a at 0.8 MPa and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 1.2 MPa and 40°C. Now the valve is opened, and the refrigerant is allowed to enter the tank. The valve is closed when it is observed that the tank contains saturated liquid at 1.2 MPa. Determine the mass of the refrigerant that has entered the tankarrow_forwardDetermine the quality of steam at 169.06 kPa when 270 kJ/kg of energy are lost from saturated steam. What is the steam temperature?arrow_forwardA 0.06-m3 rigid tank initially contains refrigerant- 134a at 0.8 MPa and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant- 134a at 1.2 MPa and 36°C. Now the valve is opened, and the refrigerant is allowed to enter the tank. The valve is closed when it is observed that the tank contains saturated liquid at 1.2 MPa. Determine (a) the mass of the refrigerant that has entered the tank and (b) the amount of heat transfer.arrow_forward
- Steam flows steadily through a turbine at a rate of 45,000 lbm/h, entering at 1000 psia and 900°F and leaving at 5 psia as saturated vapor. If the power generated by the turbine is 4 MW, determine the rate of heat loss from the steam.arrow_forward1.5-lbm of water at 350 psia fill a weighted piston-cylinder device whose volume is 1 ft3. The water is then heated at constant pressure until the temperature reaches 550◦F. Determine the resulting change in the water’s total entropy.arrow_forward3-kg of helium gas at 100 kPa and 27C are adiabatically compressed to 900 kPa. If the isentropic compression efficiency is 80 percent, determine the required work input and the final temperature of helium.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





