
Concept explainers
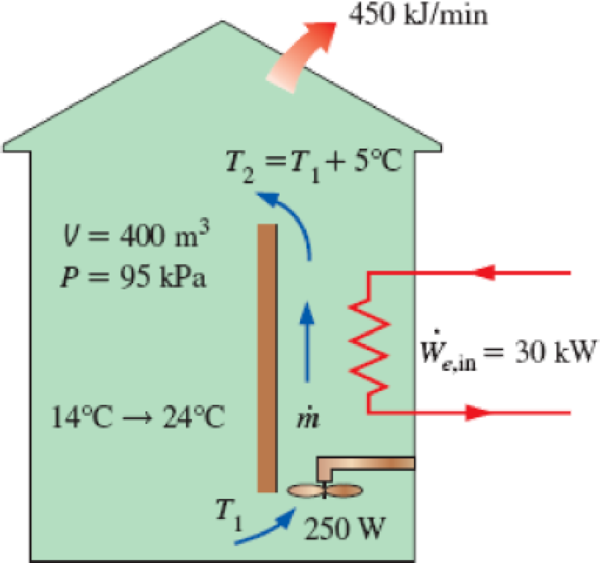
A building with an internal volume of 400 m3 is to be heated by a 30-kW electric resistance heater placed in the duct inside the building. Initially, the air in the building is at 14°C, and the local atmospheric pressure is 95 kPa. The building is losing heat to the surroundings at a steady rate of 450 kJ/min. Air is forced to flow through the duct and the heater steadily by a 250-W fan, and it experiences a temperature rise of 5°C each time it passes through the duct, which may be assumed to be adiabatic.
- (a) How long will it take for the air inside the building to reach an average temperature of 24°C?
- (b) Determine the average mass flow rate of air through the duct.
FIGURE P5–173
(a)
The time taken to attain the building’s average temperature of
Answer to Problem 188RP
The time taken to attain the building’s average temperature of
Explanation of Solution
Consider the entire building as system and the air circulates the in the building itself. There is no leakage to the surrounding.
The air flows at steady state through one inlet and one exit system (pipe and duct flow). Hence, the inlet and exit mass flow rates are equal.
Write the energy balance equation.
Here, the heat transfer is
In this system two work inputs are involved namely, the work input to the electric heater
The Equations (I) reduced as follows.
Here, there is no mass leakage from the building to the surrounding. The mass of air circulates in the building itself. Hence, inlet and exit enthalpies are neglected.
The change in internal energy is expresses as follow.
Here, the specific heat at constant volume is
Neglect the inlet and exit enthalpies and substitute
Equation (II).
Express the Equation (III) with respect to change of time and rearrange it to obtain
Write the formula for mass of air
The mass flow rate
Here, the change in time or time interval is
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant of air
Refer Table A-2, “Ideal-gas specific heats of various common gases”.
The specific heat at constant volume
Conclusion:
Substitute
Substitute
Substitute
Thus, the time taken to attain the building’s average temperature of
(b)
The average mass flow rate of air through the duct.
Answer to Problem 188RP
The average mass flow rate of air through the duct is
Explanation of Solution
Consider the heating duct with fan and heater only as the system. The air passes through in it steadily.
The system is at steady state. Hence, the rate of change in net energy of the system becomes zero.
The heating duct is an adiabatic duct. Hence, there is no heat loss.
The Equations (II) reduced as follows.
Express the Equation (VII) with respect to change of time as follows.
The change in enthalpy is expresses as follow.
Here, the specific heat at constant pressure is
Substitute
Refer Table A-2, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
It is given that the temperature rise is
Substitute
Thus, The average mass flow rate of air through the duct is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- A hair dryer is basically a duct in which a few layers of electric resistors are placed. A small fan pulls the air in and forces it through the resistors where it is heated. Air enters a 1200-W hair dryer at 100 kPa and 22°C and leaves at 47°C. The cross-sectional area of the hair dryer at the exit is 60 cm2 . Neglecting the power consumed by the fan and the heat losses through the walls of the hair dryer, determine the volume flow rate of air at the inlet.arrow_forwardA hair dryer is basically a duct in which a few layers of electric resistors are placed. A small fan pulls the air in and forces it through the resistors where it is heated. Air enters a 1200-W hair dryer at 100 kPa and 22°C and leaves at 47°C. The cross-sectional area of the hair dryer at the exit is 60 cm2 . Neglecting the power consumed by the fan and the heat losses through the walls of the hair dryer, determine the velocity of the air at the exit.arrow_forwardA 0.8-m3 rigid tank contains carbon dioxide (CO2) gas at 250 K and 100 kPa. A 500-W electric resistance heater placed in the tank is now turned on and kept on for 40 min, after which the pressure of CO2 is measured to be 175 kPa. Assuming the surroundings to be at 300 K and using constant specific heats, determine the net amount of heat transfer from the tank.arrow_forward
- Steam at 100 psia and 650F is expanded adiabatically in a closed system to 10 psia. Determine the work produced, in Btu/lbm, and the final temperature of steam for an isentropic expansion efficiency of 80 percent.arrow_forwardA student living in a 4 - m 5 - m 3 - m dormitory room turns on her 100 - W fan before she leaves the room on a summer day, hoping that the room will be cooler when she comes back in the evening. Assuming all the windows are tightly closed and disregarding any heat transfer through the walls and the windows, determine the temperature in the room when she comes back 8 h later. Use specific heat values at room temperature, and assume the room to be at 100 kPa and 20 ° C in the morning when she leaves.arrow_forwardAir enters the duct of an air-conditioning system at 15 psia and 50°F at a volume flow rate of 450 ft3 /min. The diameter of the duct is 10 in, and heat is transferred to the air in the duct from the surroundings at a rate of 2 Btu/s. Determine the velocity of the air at the duct inlet.arrow_forward
- Water is heated in an insulated, constant-diameter tube by a 7-kW electric resistance heater. If the water enters the heater steadily at 20°C and leaves at 75°C, determine the mass flow rate of water.arrow_forwardIn large compressors, the gas is often cooled while being compressed to reduce the power consumed by the compressor. Explain how cooling the gas during a compression process reduces the power consumption.arrow_forwardWhat are adiabatic and isothermal processes? Calculate the work done in adiabatic expansion during the working of a reversible heat engine.arrow_forward
- Electric heating systems used in many homes consist of a simple duct with resistance heaters. As it flows over the resistance wires, the air is heated. Consider a 25 kW electric heating system. Air enters the heating section with an air flow rate of 120 m3 / min at 100 kPa and 12 ° C. If 150 W of heat is lost from the air in the duct to the environment, determine the exit temperature of the air.arrow_forwardWhat is an adiabatic process? What is an adiabatic system?arrow_forwardA room contains 60 kg of air at 100 kPa and 15°C. The room has a 250-W refrigerator (the refrigerator consumes 250 W of electricity when running), a 120-W TV, a 1-kW electric resistance heater, and a 50-W fan. During a cold winter day, it is observed that the refrigerator, the TV, the fan, and the electric resistance heater are running continuously but the air temperature in the room remains constant. The rate of heat loss from the room that day isarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





