
(a)
The temperature of the air at the exit.
(a)
Answer to Problem 82P
The temperature of the air at the exit is
Explanation of Solution
Draw the schematic diagram for the evaporator section.
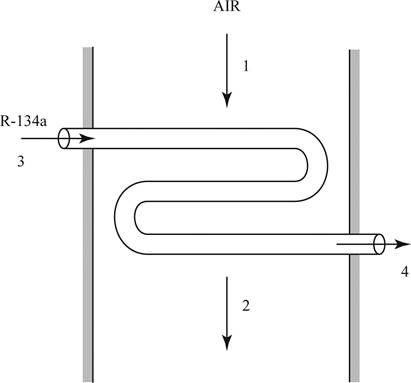
Write the formula to calculate the specific enthalpy of steam
Here, specific enthalpy of saturated liquid is
Write the ideal gas equation for specific volume of air
Here, gas constant for air is
Calculate the volume flow rate
Here, mass flow rate of air is
Write the expression for the mass balance.
Here, mass of the fluid entering into the system is
Write the energy rate balance equation for a control volume.
Here, total energy rate at inlet is
Conclusion:
Refer Table A-1E, “Gas constant of common gases”, obtain the gas constant of air as
Refer Table A-2E, “Ideal – gas specific heats of common gases”, obtain the constant pressure specific heat of air as
Refer Table A-12E, “Saturated R-134a-Pressure table”, obtain the properties of saturated R-134a at pressure
Substitute
Refer Table A-12E, “Saturated R-134a-Pressure table”, obtain the specific enthalpy
Substitute
Substitute
Rewrite Equation (I) for the mass flow rate of air
Here, mass flow rate of air at inlet is
Rewrite the Equation (I) for the mass flow rate of R-134a
Here, mass flow rate of R-134a at inlet is
Rewrite Equation (II) for the energy balance in the steam heating system.
Substitute
Here, specific enthalpy of air at the inlet is
Substitute
Thus, the temperature of the air at the exit is
(b)
The rate of heat transfer from the air to the refrigerant.
(b)
Answer to Problem 82P
The rate of heat transfer from the air to the refrigerant is
Explanation of Solution
Write the formula to calculate the rate of heat transfer from the air to the refrigerant
Conclusion:
Substitute
Thus, the rate of heat transfer from the air to the refrigerant is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





