
Concept explainers
In a single-flash geothermal power plant, geothermal water enters the flash chamber (a throttling valve) at 230°C as a saturated liquid at a rate of 50 kg/s. The steam resulting from the flashing process enters a turbine and leaves at 20 kPa with a moisture content of 5 percent. Determine the temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of the flash chamber is (a) 1 MPa, (b) 500 kPa, (c) 100 kPa, (d) 50 kPa.
FIGURE P5–186
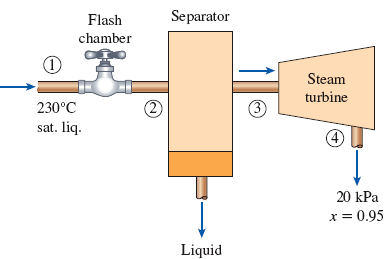
(a)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
Answer to Problem 186RP
The exit temperature of flash chamber is
The power output turbine is
Explanation of Solution
Draw schematic diagram of single flash geothermal power plant as shown in Figure 1.
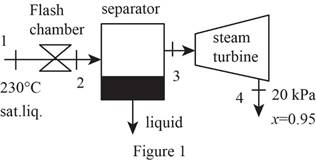
Write the general energy rate balance equation.
Here, the rate of total energy in is
Consider the system operates at steady state. Hence, the rate of change in net energy of the system becomes zero.
The Equation (I) is reduced as follows.
Refer Figure 1.
The flash chamber is nothing but the expansion valve. At expansion valve, the enthalpy kept constant.
Express the energy balance equation for the flash chamber.
Express the energy balance equation for the separator.
Express the energy balance equation for the turbine.
At state 1:
The geothermal water is extracted at the state of saturated liquid at the temperature of
The enthalpy at state 1 is as follows.
Refer Table A-4, “Saturated water-Temperature table”
The enthalpy
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
At state 2:
The exit pressure of the flash chamber is
The geothermal steam is flashed at constant enthalpy. The exit steam of the flash chamber is at the quality of
Here, the fluid enthalpy is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 4:
The steam is at the state of saturated mixture at the pressure of
The quality at state 4 is as follows.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
Write the formula for mass flow rate of vapor at entering the turbine.
Conclusion:
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (V).
Substitute
Equation (III).
Thus, the power output turbine is
(b)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
Answer to Problem 186RP
The exit temperature of flash chamber is
The power output turbine is
Explanation of Solution
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
Conclusion:
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
(c)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
Answer to Problem 186RP
The exit temperature of flash chamber is
The power output turbine is
Explanation of Solution
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
Conclusion:
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
(d)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
Answer to Problem 186RP
The exit temperature of flash chamber is
The power output turbine is
Explanation of Solution
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
Conclusion:
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- In a power plant, water vapor with a flow rate of 35 kg / s enters the adiabatic turbine at a pressure of 10 MPa and a temperature of 500° C, and comes out at a pressure of 150 kPa and 92% dryness at a speed of 200 m / s. The velocity at the turbine inlet is negligibly small compared to the outlet. The water coming out of the turbine is cooled in the condenser by cooling water whose inlet temperature is 20° C. At the condenser outlet, the cooling water temperature rises to 40 ° C, the steam pressure is 100 kPa, the temperature is 60 ° C. Coolant can be regarded as compressed liquid.a) Power generated in the turbineb)Heat transferred to the cooling water in the condenserc)Calculate the cooling water flowarrow_forwardRefrigerant-134a enters an adiabatic compressor as saturated vapor at 160 kPa at a rate of 2 m3 /min and is compressed to a pressure of 900 kPa. Determine the minimum power that must be supplied to the compressor.arrow_forwardSteam at 1000 kPa, a temperature of 300°C, and a velocity of 50 m/s. The steam leaves the turbine at a pressure of 150 kPa and a velocity of 200 m/s. Determine the work per kg of steam flowing through the turbine, assuming the process to be reversible and adiabatic.arrow_forward
- Refrigerant-134a enters an adiabatic compressor as saturated vapor at 30 psia at a rate of 20 ft3 /min and exits at 70 psia pressure. If the isentropic efficiency of the compressor is 80 percent, determine the second-law efficiency of the compressor. Assume the surroundings to be at 75°F.arrow_forwardRefrigerant-134a at 700 kPa and 120°C enters an adiabatic nozzle steadily with a velocity of 20 m/s and leaves at 400 kPa and 30°C. Determine (a) the exit velocity and (b) the ratio of the inlet to exit area A1/A2.arrow_forwardRefrigerant-134a enters an adiabatic compressor as saturated vapor at 30 psia at a rate of 20 ft3 /min and exits at 70 psia pressure. If the isentropic efficiency of the compressor is 80 percent, determine the actual power input.arrow_forward
- Water is heated in an insulated, constant-diameter tube by a 7-kW electric resistance heater. If the water enters the heater steadily at 20°C and leaves at 75°C, determine the mass flow rate of water.arrow_forwardWater at 80°F and 20 psia is heated in a chamber by mixing it with saturated water vapor at 20 psia. If both streams enter the mixing chamber at the same mass flow rate, determine the temperature and the quality of the exiting stream.arrow_forwardAir enters the turbine at 1 MPa and 327 degree* C with a velocity of 100 m/s and exits at 100 kPa and 27 degree*C with a low velocity. Heat transfer loss from the turbine surface is 1200kJ / min and the power output of the turbine is 240 kW. Determine the mass flow rate of air through the turbine.arrow_forward
- A 0.05-m3 rigid tank initially contains refrigerant134a at 0.8 MPa and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 1.2 MPa and 40°C. Now the valve is opened, and the refrigerant is allowed to enter the tank. The valve is closed when it is observed that the tank contains saturated liquid at 1.2 MPa. Determine the mass of the refrigerant that has entered the tankarrow_forwardLiquid water at 200 kPa and 20 degress celcius is heated in a chamber by mixing it with superheated steam at 200 kPa and 250 degrees celcius. Liquid water enters the mixing chamber at a rate of 4.2 kg/s, and the chamber is estimated to lose heat to the surrounding air at 23 degress celcius at a rate of 1200 kJ/min. If the mixture leaves the mixing chamber at 200 kPa and 85 degrees celcius determine (a) the mass flow rate of the superheated steam, and (b) the rate of entropy generation during the mixing process.arrow_forwardSteam enters a turbine with an enthalpy of 3300 KJ/kg and leaves with an enthalpy of 2400 kJ/kg. The power output of the steam turbine is 6 MWarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





