
Refrigerant-134a at 1 MPa and 90°C is to be cooled to 1 MPa and 30°C in a condenser by air. The air enters at 100 kPa and 27°C with a volume flow rate of 600 m3/min and leaves at 95 kPa and 60°C. Determine the mass flow rate of the refrigerant.
FIGURE P5–81
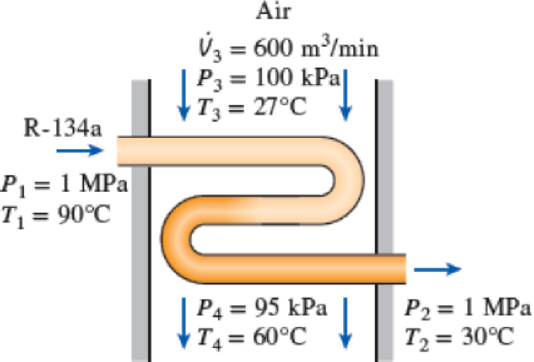
The mass flow rate of the refrigerant.
Answer to Problem 81P
The mass flow rate of the refrigerant is
Explanation of Solution
Consider the system is in steady state. Hence, the inlet and exit mass flow rates are equal.
The mass flow rate of air
The mass flow rate of refrigerant
Write the energy rate balance equation for one inlet and one outlet system.
Here, the rate of heat transfer is
The system is at steady state. Hence, the rate of change in net energy of the system becomes zero.
Neglect the work transfer, heat transfer to the surrounding, potential and kinetic energies.
The Equations (I) reduced as follows for air.
The Equations (I) reduced as follows for refrigerant.
Combining Equation (II) and (III).
Substitute
Write the formula for change in enthalpy
Substitute
For refrigerant:
At inlet:
The refrigerant is at the state of superheated condition.
Refer Table A-13, “Superheated refrigerant-134a”.
Obtain the inlet enthalpy
At exit:
The refrigerant is at the state of saturated liquid.
Refer Table A-11, “Saturated refrigerant-134a-Temperature table”.
Obtain the exit enthalpy
For air:
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant of air
Refer Table A-2, “Ideal2gas specific heats of various common gases”.
The specific heat at constant pressure
Write the formula for mass flow rate of air
Here, the volumetric flow rate of air is
Conclusion:
Substitute
Substitute
Thus, the mass flow rate of the refrigerant is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
- Refrigerant-134a is to be cooled by water in a condenser. The refrigerant enters the condenser with a mass flow rate of 6 kg/min at 1 MPa and 70°C and leaves at 35°C. The cooling water enters at 300 kPa and 15°C and leaves at 25°C. Neglecting any pressure drops, determine the mass flow rate of the cooling water required.arrow_forwardAir enters the compressor of a gas turbine plant at ambient conditions of 100 kPa and 25 ⁰C with a low velocity and exits at 1 MPa and 347 ⁰C with a velocity of 90 m/s. The compression process is adiabatic, and the power input is 271. Determine the mass flowrate of the air through the compressor.arrow_forwardSteam enters a turbine steadily at a flow rate of 1 kg/s at 7 MPa and 500 degrees and exits as saturated steam at 40 kPa. If there is a heat loss of 10 kW from the turbine, what will be the power produced by the turbine?arrow_forward
- Superheated steam enters the turbine of a steam power plant at 5 MPa and 450 ºC, and exits at 10 kPa. Determine the actual turbine work when the turbine isentropic efficiency is 90% and the mass flow rate of steam is 10 kg/s.arrow_forwardAir enters the compressor of a gas-turbine plant at ambient conditions of 100 kPa and 30°C with a low velocity and exits at 1 MPa and 367 °C with a velocity of 6×103 m/min. The compressor is cooled at a rate of 1800 kJ/min, and the power input to the compressor is 250 kW. Determine the mass flow rate of air through the compressor.arrow_forwardRefrigerant-134a enters an adiabatic compressor as saturated vapor at 100 kPa at a rate of 0.7 m3 /min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 87 percent, determine the power input, in kW.arrow_forward
- Steam at 1000 kPa, a temperature of 300°C, and a velocity of 50 m/s. The steam leaves the turbine at a pressure of 150 kPa and a velocity of 200 m/s. Determine the work per kg of steam flowing through the turbine, assuming the process to be reversible and adiabatic.arrow_forwardRefrigerant-134a at 1 MPa and 90°C is to be cooled to 1 MPa and 30°C in a condenser by air. The air enters at 100 kPa and 27°C with a volume flow rate of 600 m3/min and leaves at 95 kPa and 60°C. Determine the mass flow rate of the refrigerant.arrow_forwardRefrigerant-134a is to be cooled by water in a condenser. The refrigerant enters the condenser with a mass flow rate of 6 kg/min at 1 MPa and 70°C and leaves at 35°C. The cooling water enters at 300 kPa and 15°C and leaves at 25°C. Neglecting any pressure drops, determine the heat transfer rate from the refrigerant to water.arrow_forward
- Refrigerant-134a enters a compressor at 100 kPa and –24°C with a flow rate of 1.35 m3 /min and leaves at 800 kPa and 60°C. Determine the mass flow rate of R-134a and the power input to the compressor.arrow_forwardRefrigerant-134a enters an adiabatic compressor as saturated vapor at 100 kPa at a rate of 0.7 m3 /min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 87 percent, determine the temperature of the refrigerant at the exit of the compressor.arrow_forwardSteam enters an adiabatic turbine steadily at 3 MPa and 400°C and leaves at 50 kPa and 100°C. If the power output of the turbine is 2 MW, determine the isentropic efficiency of the turbine.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





