
(a)
Interpretation:
The major product for the given reaction has to be determined.
Concept introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Carbocation: it is carbon ion that bears a positive charge on it.
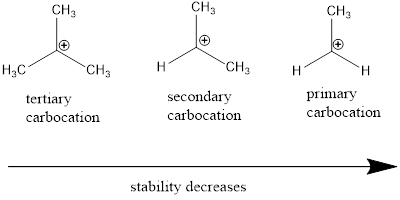
Carbocation stability order:
(b)
Interpretation:
The major product for the given reaction has to be determined.
Concept introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(c)
Interpretation:
The major product for the given reaction has to be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(d)
Interpretation:
The major product for the given reaction has to be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Addition of halogen to an alkene: The addition of halogen to an alkene compound forms cyclic 3 membered intermediate as the first step which then the leads to the product formation. Example for this is as follows,
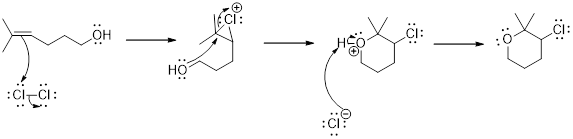
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





