
Concept explainers
(a)
Interpretation:
The product obtained from reaction
Concept Introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition of hydrogen halides to
Electrophilic addition of hydrogen halide to alkyne occurs according to the following general mechanism.
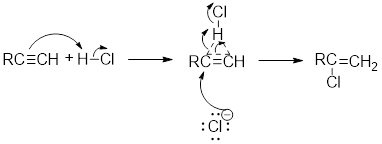
First a
(b)
Interpretation:
The product obtained from reaction
Concept Introduction:
Addition of hydrogen halides to alkynes:
Electrophilic addition of hydrogen halide to alkyne occurs according to the general mechanism.
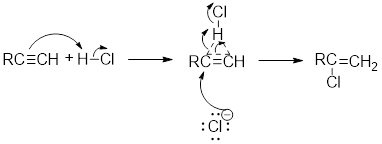
First a
(c)
Interpretation:
The product obtained from reaction
Concept Introduction:
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a
Conversion of terminal alkynes into enol: If we want to convert terminal alkyne into an enol, the presence of mercuric ion as a catalyst should be needed and the catalyst will increase the
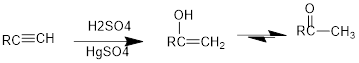
(d)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagents is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans
(e)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagent is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
(f)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagents is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw the major organic product for the reaction between 1,2-dimethylcycloheptene and a cold, dilute solution of KMnO4.arrow_forwardChoose the appropriate reagent for the following reaction. A) TsCl/pyridine B) SOCl2/pyridine C) ZnCl2 D) PBr3 E) HIarrow_forwardWhat reagents would you use to prepare the next alkenearrow_forward
- Choose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forwardChoose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) KMnO4, H3O+ CH3Cl, AlCl3 HNO3, H2SO4 Cl2, FeCl3 fuming sulfuric acidarrow_forward1. Which reaction conditions would be best for turning ethene into ethanol? a.H2O, HBr b. CH3OH, H2SO4 c.CH3OH, HBR d. H2O, H2SO4 2. Which alkene would be non-regioselective in a reaction with HBr? a. 2-ehyl-2-methyl-2-butene b.2-ethyl-3-methyl-2-butene c. 2,3-dimethyl-2-butene d.1,2-dimethyl-2-butenearrow_forward
- Draw the structure of the product of the reaction between the compound shown below and H2SO4.arrow_forwardThe following series of reactions yields ___________. 1) Butanoic acid and SOCl2; set product aside for use in step 3 2) Bromoethane in excess plus Mg and ether 3) Add the product of step 1 to the reaction mix of step 2 4) When the reaction is complete, add dilute aqueous acid 3-ethylhexan-3-ol 3-ethylheptan-2-ol hexanoic acid hexanal 3-heptanonearrow_forwardWhat is the major product of the reaction of 1 mol of propyne with each of the following reagents? a. HBr (1 mol) e. aqueous H2SO4, HgSO4 h. H2/Lindlar catalyst b. HBr (2 mol) f. R2BH in THF followed by i. sodium amide c. Br2 (1 mol)/CH2Cl2 H2O2/HO-/H2O j. the product of part i followed by d. Br2 (2 mol)/CH2Cl2 g. excess H2, Pd/C 1-chloropropanearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


