
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
2nd Edition
ISBN: 9780137533114
Author: Dean Appling, Spencer Anthony-Cahill
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 3P
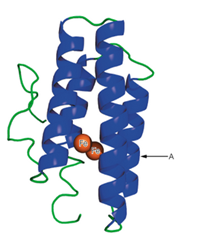
A schematic structure of the subunit of hemerythrin (an oxygen-binding protein from invertebrate animals) is shown to the right.
a. It has been found that in some of the a-helical regions of hemerythrin, about every third or fourth amino acid residue is a hydrophobic one. Suggest a structural reason for this finding.
b. What would be the effect of a mutation that placed a proline residue at point A in the structure?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
i. A schematic structure of the subunit of hemerythrin (an oxygen-binding
protein from invertebrate animals) is shown to the right.
(a) It has been found that in some of the a-helical regions of hemerythrin,
about every third or fourth amino acid residue is a hydrophobic one.
Suggest a structural reason for this finding.
(b) What would be the effect of a mutation that placed a proline residue at
point A in the structure?
In the molecule of oligomeric protein there are
19 lysine residues. 12 of them may be easily
acetylated with anhydrides of dicarbon acids
(it react with NH2-groups). The acetylation of
extra two residues of lysine will dissociate the
protein to the subunits. The rest 5 lysine
residues may be modified only after
denaturation of the protein. Suggest, how
many lysine residues are:
a) on a surface of protein globule;
b) inside globule:
c) in a site which is responsible for the contact
within subunits
Consider a short peptide that forms an alpha-helix within a larger protein structure. Suppose that one glutamate residue at some specific position in the helix were mutated to a leucine residue. The mutation could either make the helix more stable, or less stable.
i) Describe two situations in which a Glu-to-Leu mutation could make the helix more stable.
ii) Describe two situations in which the Glu-to-Leu mutation could make the helix less stable.
Explain briefly the basis for the stabilizing and destabilizing effect in all cases.
Chapter 6 Solutions
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
Ch. 6 - Prob. 1PCh. 6 - Bovine pancreatic trypsin inhibitor (BPTI; Figure...Ch. 6 - A schematic structure of the subunit of...Ch. 6 - In the protein adenylate kinase, the C-terminal...Ch. 6 - Give two reasons to explain why a proline residue...Ch. 6 - Consider a small protein containing 101 amino acid...Ch. 6 - a. Based on a more conservative answer to Problem...Ch. 6 - The following sequence is part of a globular...Ch. 6 - a. A protein is found to be a tetramer of...Ch. 6 - Under physiological conditions, the protein...
Ch. 6 - Theoretical and experimental measurements show...Ch. 6 - The peptide hormone vasopressin is used in the...Ch. 6 - A protein gives under conditions of buffer...Ch. 6 - A protein gives a single band on SDS get...Ch. 6 - It has been postulated that the normal...Ch. 6 - Below are shown two views of the backbone...Ch. 6 - Do you expect a Pro Gly mutation in a...Ch. 6 - Rank the following in terms of predicted rates...Ch. 6 - Shown below are two cartoon views of the small...Ch. 6 - Prob. 20PCh. 6 - In most cases, mutations in the core of protein...Ch. 6 - A Leu Ala mutation at a site buried the core of...Ch. 6 - Disulfide bonds have been shown to stabilize...Ch. 6 - Cartoon renderings of the proteins Top 7 and adaH2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- A protein hypothetically consists of two polypeptide chains with the given sequences in the picture. Based on the said sequences, a. Do you think it is possible that interchain disulfide bonds is present in the protein? Explain. b. Do you think it is possible that intrachain disulfide bonds is present in the protein? Explain.arrow_forwardPatients suffering from sickle cell anemia have a mutation in the gene that codes for one of the hemoglobin chains, in which a single glutamate is replaced by a valine. Propose an explanation for why this substitution has such a striking effect on protein structure and function with explanation. Suggest two other amino acids that would be less likely to cause such serious impairment of hemoglobin function if each was substituted for glutamate. Briefly explain your answer.arrow_forwardThe endorphins are a group of naturally occurring neurotransmitters that act in a manner like morphine to control pain. Research has shown that the biologically active parts of the endorphin molecules are simple pentapeptides called enkephalins. Draw the structure of the methionine enkephalin with the sequence Tyr-Gly-Gly-Phe-Met. Identify the N-terminal and C-terminal amino acids. Bradykinin is a nonapeptide that stimulates smooth muscle contraction, dilates blood vessels, causes pain, and is a component of bee venom. Give the IUPAC name of the nonapeptide. Note: The selected photo is the sequence of Bradykinin.arrow_forward
- A recent genome sequencing project for the bacterium Burkholderia mallei has identified a new protein with high similarity to the lysylphosphatidylglycerol flippase enzyme. A short section of the new protein sequence is shown below. TVEVNAPGDVQKALSELQQINDGRLDIRI (a) Are any reverse turns likely to be present? Explain your answer. (b) Are any beta-strands likely to be present? Explain your answer. (c) Are any alpha helices likely to be present? Explain your answer. (d) Is any supersecondary structure likely to be present? Explain your answer. (e) Identify two residues that are likely to be buried in the core of the folded protein. Explain your answer. (f) Identify two residues that are likely to be hydrogen bonded to each other. Explain your answer.arrow_forwardA synthetic polypeptid made up of L-glutamic acid residues is in a random coil configuration at pH 7.0 but changes to alpha helical when the pH is lowered to 2.0. Explain this pH-dependent conformational transition.arrow_forward-. A schematic structure of the subunit of hemerythrin (an oxygen- binding protein from invertebrate animals) is shown below. (a) It has been found that in some of the a-helical regions of hemerythrin, about every third or fourth amino acid residue is a hydrophobic one. Suggest a structural reason for this finding. (b) What would be the effect of a mutation that placed a proline residue at point A in the structure? A Fearrow_forward
- To better understand the effects of palmitoylation on protein X, researchers want to chemically attach a palmitic acid at a specific position in this protein. The researchers first try to feed cells with palmitic acid or an amino acid with a C16 side chain. Neither of the initial experiments is successful in helping the researchers learn more about the biological role of palmitoylated protein X. Why? Suggest a strategy to specifically incorporate a palmitoylation site into protein X.arrow_forwardGive two reasons to explain why a proline residue in the middle of an αhelix is predicted to be destabilizing to the helical structure.arrow_forwardFor the peptide Ala-Cys-His-Ile-Leu-Asp a. Write the single letter code for the amino acid residues b. What is the charge of the peptide at pH 7.0. Assign the following pKa values: 3,4,6,8,9 c. What is the pI of the peptidearrow_forward
- Certain polypeptide sequences that show a pronounced tendency to dimerize in an antiparallel coiled-coil structure exhibit an exact repetition of leucine every 7 residues. If you also know that the a helix is distorted somewhat from its usual 3.6 residues/turn in coiled coils, propose a mechanism for the dimerization. What does this suggest that the residue/turn value might be in a coiled coil?arrow_forwardConsidering the chemical characteristics of the amino acids valine and glutamic acid (see Figure 5.14), propose a possible explanation for the dramatic effect on protein function that occurs when valine is substituted for glutamic acid.arrow_forwardEstimate the length, in nm, of the four identical beta-strands drawn in blue in panel (B) of the image below, if they consists of only the amino residues of the two alpha-helices highlighted in purple in panel (A) of the image below. Assume that the amino acid residues of the two alpha-helices combined were folded in four beta-strands of identical length.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Mitochondrial mutations; Author: Useful Genetics;https://www.youtube.com/watch?v=GvgXe-3RJeU;License: CC-BY