
Concept explainers
Consider the following energy diagram.
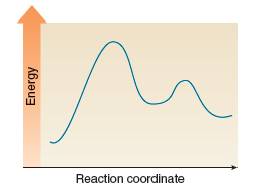
(a) How many steps are involved in this reaction?
(b) Label
(c) Label each transition state.
(d) Which point on the graph corresponds to a reactive intermediate?
(e) Which step is rate-determining?
(f) Is the overall reaction endothermic or exothermic?
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
LL ORG CHEM
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties
Organic Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Principles of Chemistry: A Molecular Approach (3rd Edition)
- (a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion.Reaction rates increase with increasing temperature.The slowest step in a reaction is called the rate-determining step.Catalysts increase reaction rates.The fastest step in a reaction is called the rate-determining step.As a reaction progresses its rate goes down.Reaction rates can show little change as masses of solid reactants increase.arrow_forwardConsider the following reaction: (a) The rate law for this reaction is first order in HBr(g) and first order in O₂(g). What is the rate law for this reaction? Rate = k [HBr(g)] [O₂(g)] O Rate = k [HBr(g)]² [O₂(g)] O Rate = k [HBr(g)] [O₂(g)]² O Rate = k [HBr(g)]² [0₂(g)]² O Rate = k [HBr(g)] [O₂(g)]³ O Rate = k [HBr(g)]4 [0₂(g)] (b) If the rate constant for this reaction at a certain temperature is 11500, what is the reaction rate when [HBr(g)] = 0.00379 M and [O₂(g)] = 0.00876 M? Rate = 4 HBr(g) + O₂(g) → 2 H₂O(g) + 2 Br₂(g) M/s. Rate = (c) What is the reaction rate when the concentration of HBr(g) is doubled, to 0.00758 M while the concentration of O₂(g) is 0.00876 M? M/Sarrow_forward4. A) The enzyme urease catalyzes the hydrolysis of urea to ammonia and carbon dioxide. The uncatalyzed reaction has an activation energy of 125 kJ/mol. The enzyme catalyzes a mechanism that has an activation energy of 46 kJ/mol. By what factor does urease increase the rate of urea hydrolysis at 21 celsius? B) True or False: A catalyst increases the rate of a reaction by changing the mechanism of the reaction such that there is a different rate law for the reaction when a catalyst is present. C) True or False: A catalyst increases the equilibrium constant of a reaction and therefore makes the products more favored. D) True or False: In order for a catalyst to function it must exist in the same phase as the substrate.arrow_forward
- Consider the following reaction: (a) The rate law for this reaction is first order in NO₂(g) and first order in O3(g). What is the rate law for this reaction? O Rate = k [NO₂(g)] [03(9)] Rate = k [NO₂(g)]² [03(9)] O Rate = k [NO₂(g)] [03(9)]² O Rate = k [NO₂(g)]² [03(g)]² Rate = k [NO₂(g)] [03(g)]³ Rate = k [NO₂(g)]4 [03(9)] (b) If the rate constant for this reaction at a certain temperature is 73200, what is the reaction rate when [NO₂(g)] = 0.973 M and [O3(9)] = 1.42 M? Rate = 2 NO₂(g) + 03(g) → N₂05(9) + O₂(g) M/s. Rate = (c) What is the reaction rate when the concentration of NO₂(g) is doubled, to 1.95 M while the concentration of O3(g) is 1.42 M? M/sarrow_forwardThe graph below is a plot of the distribution or fraction of molecules having certain kinetic energies. These molecules can undergo reaction with an activation energy of Ea. (A) Which region of the graph (1, 2, 3, or 4) corresponds to the fraction of molecules with enough energy to react? (B) Which region of the graph corresponds to the fraction of molecules that do not have enough energy to react?arrow_forward⦁ 4. Make your own energy diagram for an exothermic reaction that does not include a catalyst and one with a catalyst (Be sure each line is a different color or labeled). Label the axis of the diagram. Then, state what a catalyst is and how a catalyst affects the reaction rate.arrow_forward
- Given the following balanced equation, determine the rate of reaction with respect to [H,]. (6HNZ - (6He + (0°N O Rate 2 4H2 O Rate # - O Rate O Rate = O tis not possible to determine the answer without more information.arrow_forwardFrom "Factors affecting the rate of the reaction."arrow_forwardii) Explain the effect of Catalyst on the rate of a chemical reaction.arrow_forward
- a.)Label the following energy diagram with activated complex, activation energy, product energy, reactant energy b.)Draw the effect of a catalysts on the diagram c.)Is the reaction drawn endothermic or exothermic? How do you know?arrow_forwardWhich of the following is true given the information below? 3 H₂ (g) + N₂ (g) → 2NH₃ (g); ∆H = -22 kcal/mol A) The reaction is endothermic. B) The product is higher in energy than the reactants. C) Heat is released D) The activation energy for the reaction is negative.arrow_forwardThe graph in Figure 20-2 shows energy changes that take place when hydrogen and iodine react to form hydrogen iodide during an uncatalyzed reaction. Energy (1) 1000+ H₂ 12 500+ co+●● 100+ H₂(g) + 1₂(g) = 2 HI(g) Time Figure 20-2 HIHIarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





