
To determine : The minimum number of chlorine (Cl) atoms needed for the given reaction.
Introduction : The reaction between methane and chlorine gas results in the formation of HCl and CCl4. When scientists write
Answer to Problem 7STP
D. 8
Explanation of Solution
In a chemical reaction, matter cannot be created or destroyed. This is law of conservation of mass. Therefore, the equations must be balanced to show balance of mass. The number of atoms of each element on the reactant side must be equal to the number of atoms of the same element on the product side. Coefficients are used to balance the number of atoms. In the case of given reaction, we can see that the coefficient of chlorine is 4. So, we multiply 4 with the subscript of chlorine; i.e., 2. This gives 8 atoms of chlorine. Hence, the minimum number of chlorine atoms needed for the reaction is 8.
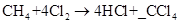
- 1- The coefficient of chlorine in the equation is 4. So there are 8 atoms of chlorine. Hence, this is not a correct option.
- 2- The coefficient of chlorine in the equation is 4. So there are 8 atoms of chlorine. Hence, this is an incorrect option.
- 4- The coefficient of chlorine in the equation is 4. So there are 8 atoms of chlorine. Hence, this is an incorrect option.
Chapter 7 Solutions
Biology Illinois Edition (Glencoe Science)
Additional Science Textbook Solutions
Microbiology: An Introduction
Microbiology with Diseases by Body System (4th Edition)
Campbell Biology in Focus (2nd Edition)
Campbell Essential Biology (6th Edition) - standalone book
Fundamentals of Anatomy & Physiology (11th Edition)
Microbiology with Diseases by Body System (5th Edition)
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





