
Concept explainers
To determine : The correct coefficient for HCl in the given chemical reaction.
Introduction : The reaction between methane and chlorine gas results in the formation of HCl and CCl4. When scientists write
Answer to Problem 6STP
C. 4 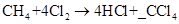
Explanation of Solution
In a chemical reaction, matter cannot be created or destroyed. This is law of conservation of mass. Therefore, the equations must be balanced to show balance of mass. The number of atoms of each element on the reactant side must be equal to the number of atoms of the same element on the product side. Coefficients are used to balance the number of atoms. In the case of given reaction, we can see that there are 4 hydrogen atoms in methane. To balance the number of hydrogen atoms, we must put a coefficient of 4 before HCl.
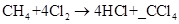
A. 1- If we put 1 as a coefficient before HCl, the number of hydrogen atoms in reactant is 4 and in product it will be only 1. So this will be an unbalanced. Hence, this is not a correct option.
B. 2- If we put 2 as a coefficient before HCl, the number of hydrogen atoms in reactant is 4 and in product it will be only 2. So this will be an unbalanced equation. Hence, this is an incorrect option.
D. 8- If we put 8 as a coefficient before HCl, the number of hydrogen atoms in reactant is 4 and in product it will be 8. So this will be an unbalanced equation. Hence, this is an incorrect option.
Chapter 7 Solutions
Biology Illinois Edition (Glencoe Science)
Additional Science Textbook Solutions
Human Anatomy & Physiology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Fundamentals of Anatomy & Physiology Plus Mastering A&P with eText - Access Card Package (10th Edition) (New A&P Titles by Ric Martini and Judi Nath)
Campbell Essential Biology with Physiology (6th Edition)
Fundamentals of Anatomy & Physiology (11th Edition)
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





