
a)
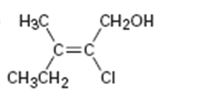
Interpretation:
To assign E or Z configuration for the compound given.
Concept introduction:
The two groups attached to the carbons in double bond are to be ranked first. The member that ranks higher can be determined by considering the
To assign:
The configuration for the compound given as E or Z.
b)
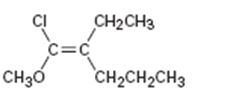
Interpretation:
To assign E or Z configuration for the compound given.
Concept introduction:
The two groups attached to the carbons in double bond are to be ranked first. The member that ranks higher can be determined by considering the atomic number of the first atom of the two substituents separately. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms away from double bond are considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms. The isomer that has the higher ranked groups on each carbon on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on the opposite sides, the alkene is said to have E configuration.
To assign:
The configuration for the compound given as E or Z.
c)
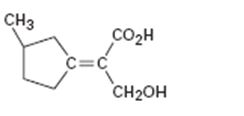
Interpretation:
To assign E or Z configuration for the compound given.
Concept introduction:
The two groups attached to the carbons in double bond are to be ranked first. The member that ranks higher can be determined by considering the atomic number of the first atom of the two substituents separately. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms away from double bond are considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms. The isomer that has the higher ranked groups on each carbon on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on the opposite sides, the alkene is said to have E configuration.
To assign:
The configuration for the compound given as E or Z.
d)
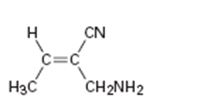
Interpretation:
To assign E or Z configuration for the compound given.
Concept introduction:
The two groups attached to the carbons in double bond are to be ranked first. The member that ranks higher can be determined by considering the atomic number of the first atom of the two substituents separately. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms away from double bond are considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms. The isomer that has the higher ranked groups on each carbon on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on the opposite sides, the alkene is said to have E configuration.
To assign:
The configuration for the compound given as E or Z.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry
- 9. Which of the following alkenes has a Z configuration?arrow_forwardName the alkene below.Use ONLY E/Z designators to indicate stereochemistry.H2C=C=CHCH3arrow_forwardName (including E/Z stereochemistry) the five alkenes that can produce 3-bromo-3-methylhexane on reaction with HBr. Draw the skeletal structure of each molecule.arrow_forward
- Bogorol A is a natural product with the potential to fight antibiotic-resistant bacteria. Shown below is an intermediate that was used in a synthesis of bogorol A. Assign the configuration of the alkene unit as either Z or E. a. Z b. Earrow_forwardName the alkene below.Use only E/Z designators to indicate stereochemistry.arrow_forwardDraw a structural formula for the most stable carbocation with each molecular formula. Q.) C4H9+arrow_forward
- ||| IV Identify the least stable carbocation. A) I B) II C) III D) IVarrow_forwardDraw the structure(s) of all of the alkene isomers, C5H10, that contain a branched chain. Consider E/Z stereochemistry of alkenes.arrow_forwarddraw the two possible carbocations that can form when this alkene reacts with a strong acid (such as HBr or H3O+). of the two structures you drew, circle the more stable carbocationarrow_forward
- 1.What molecular orbital react to form cyclohexene? 2.Draw the electrocyclic product and indicate if the substituents are cis- or con-rotary Darrow_forwardUsing cis- and trans-hex-3-ene, demonstrate that the addition of HCl is not a stereospecic reaction. Draw the structure of the stereoisomers formed from each alkene.arrow_forwardUsing cis- and trans-hex-3-ene, demonstrate that the addition of HCl is not a stereospecific reaction. Draw the structure of the stereoisomers formed from each alkene.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

