
Concept explainers
(a)
Interpretation:
The mechanism for the given reaction has to be given.
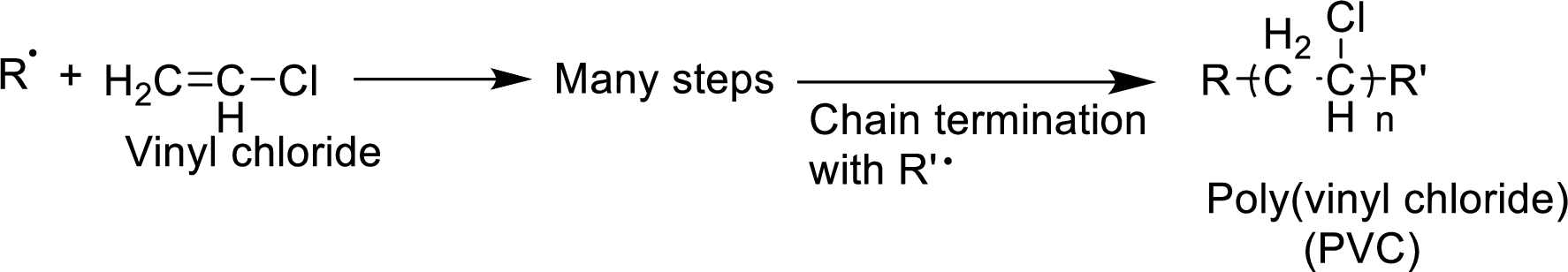
Concept Introduction:
The
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
(b)
Interpretation:
The mechanism for the formation of poly(styrene) from styrene has to be given and at which end of the styrene double bond, the
Concept Introduction:
The polymers are formed from the repetition monomer units. The polymerization process occurs in three steps.
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
- Define the Mechanism of the Radical Addition of HBr to an Alkene ?arrow_forwardImagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) When chlorobenzene reacts with a hot solution of sulfuric and nitric acids, an interesting result occurs: the reaction is slower than the reaction of the solution with benzene – which is similar to the result of the reaction of the solution with nitrobenzene – but it generates products with the same substitution pattern seen in the reaction of the solution with phenol. Explain why chlorobenzene has this curious reactivity.arrow_forwardOutline the step-by-step method (initiation, propagation(s), and one termination step) for bromination of 2-bromopropane to produce its major product.arrow_forward
- write the free radical monosubtitution mechanism for the bromination of ethane to produce bromoethanearrow_forwardWrite structural formulas for toluene (C6H5CH3) and for benzoic acid (C6H5CO2H) (a) as resonance hybrids of two Kekulé forms and (b) with the Robinson symbol.arrow_forwardAlkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C4H9)3SnH, in the presence of light (hv). Propose a radical chain mechanism by which the reaction might occur. The initiation step is the light-induced homolytic cleavage of the Sn-H bond to yield a tributyltin radical.arrow_forward
- The aerobic oxidation of para-xylene to terephthalic acid is an important process in industrial chemistry. Discuss why the oxidation of the second methyl group requires harsher conditions than the oxidation of the first methyl group. You should accurately reference all your bibliographic material.arrow_forwardThe rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forwardGive the order of reactivity of carboxylic acid and its derivatives. What is thesignificance of this order of reactivity in predicting their interconversions?arrow_forward
- Provide the mechanisms for chlorination of toluene which include the initiation, propagation and termination stepsarrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forwardChapter #6, Question #5: Suggest a possible sequence of reactions (beginning with oxidation via the hydroxyl radical) by which dimethyl sulfide ((CH3)2S) can be oxidized to produce a sulfuric acid aerosol.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



