
Interpretation:
The Lewis structure, molecular shape, bond angle and hybrid orbitals in
Concept introduction:
VSEPR theory stands for Valence Shell Electron Pair Repulsion Theory. It helps to predict the molecular shape or geometry of the molecule with the help of the number of bond pairs or lone pairs present in it.
According to the VSEPR theory, the presence of lone pair on the central atom of a molecule causes deviation from standard molecular geometry. Thus, valence electrons provide a Lewis structure, which gives an idea about electron pair geometry and hybridization.
Answer to Problem 67SSC
| Lewis structure | molecular shape, | bond angle | hybrid orbitals | |
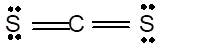 |
Linear | 180° | sp hybrid orbitals | |
 |
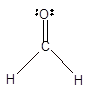
Trigonal planer | 120° | sp2 hybrid orbitals | |
 |
Bent | 104° | sp3 hybrid orbitals | |
 |
Tetrahedral | 109° | sp3 hybrid orbitals | |
 |
Pyramid | 107° | sp3 hybrid orbitals |
Explanation of Solution
Given information:
In the
Number of valence electron = Number of atom C (valence electron in C) + Number of atom S (valence electron in S) =
Hence the Lewis structure must be:
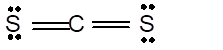
Geometry = Linear, bond angle = 180°
In the
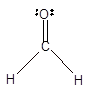
Number of valence electron = Number of atom C (valence electron in C) + Number of atom O (valence electron in O)+ Number of atom H (valence electron in H) =
Hence the Lewis structure must be:
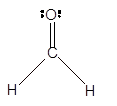
Geometry = Trigonal planer, bond angle = 120°
In the
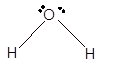
Number of valence electron = Number of atom O (valence electron in O) + Number of atom H (valence electron in H) =
Hence the Lewis structure must be:
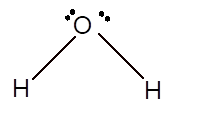
Geometry = bent, bond angle = 104°
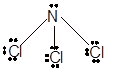
In
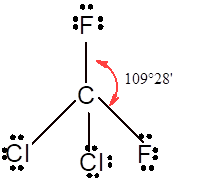
In the
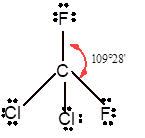
Number of valence electron = Number of atom P (valence electron in P) + Number of atom Cl (valence electron in Cl) =
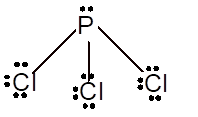
With sp3 hybridization, the geometry must be tetrahedral but the presence of one lone pair on central P atom alters the standard geometry of molecule to pyramid shape due to repulsion between lone pair and bond pair of the molecule.
Thus,
| Lewis structure | molecular shape, | bond angle | hybrid orbitals | |
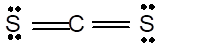 |
Linear | 180° | sp hybrid orbitals | |
 |
Trigonal planer | 120° | sp2 hybrid orbitals | |
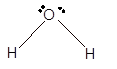 |
Bent | 104° | sp3 hybrid orbitals | |
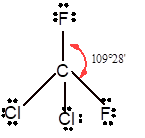 |
Tetrahedral | 109° | sp3 hybrid orbitals | |
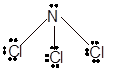 |
Pyramid | 107° | sp3 hybrid orbitals |
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (8th Edition)
Organic Chemistry (9th Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





