
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 104A
The Lewis structure of
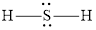
Explanation of Solution
Determine the valence electrons which are available for bonding in
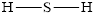
Remaining bonding pairs are
The remaining pairs-lone pair-must be added to the terminal atoms or the central atom. Hence, the Lewis structure of
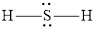
(b)
Interpretation:
Lewis structure of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 104A
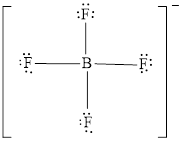
The Lewis structure of
Explanation of Solution
Determine the valence electrons which are available for bonding in
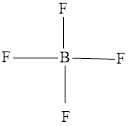
Remaining bonding pairs are
The remaining pairs-lone pair-must be added to the terminal atoms or the central atom. Hence, the Lewis structure of
(c)
Interpretation:
Lewis structure of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 104A
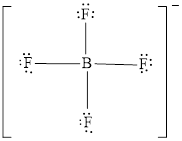
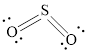
The Lewis structure of
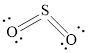
Explanation of Solution
Determine the valence electrons which are available for bonding in
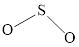
Remaining bonding pairs are
Either to the terminal atoms or the central atom, the remaining pairs that is lone pair must be added. Hence, the Lewis structure of
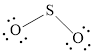
Two lone pairs from each O is taken to form double bond with S.
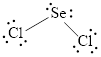
(d)
Interpretation:
Lewis structure of
Concept introduction:
Lewis structure is also called as Lewis dot structure. In this structure, valence electrons are represented by dot symbol around the central atom. This structure shows the bonding between molecule and the lone pairs which may occur in the molecule.
Answer to Problem 104A
The Lewis structure of
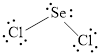
Explanation of Solution
Determine the valence electrons which are available for bonding in
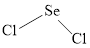
Remaining bonding pairs are
Either to the the terminal atoms or the central atom, the remaining pairs that is lone pairs must be added. Hence, the Lewis structure of
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties
Inorganic Chemistry
Organic Chemistry (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





