
Concept explainers
(a)
Draw the
(a)
Answer to Problem 180RP
The
Explanation of Solution
Draw the
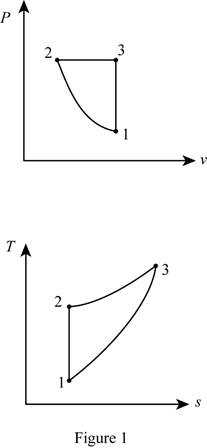
Thus, the
(b)
The expression for the back work ratio as a function of k and r.
(b)
Answer to Problem 180RP
The expression for the back work ratio as a function of k and r is
Explanation of Solution
Find the work of compression using the first law for process 1-2.
Here, heat interaction during the process 1-2 is
Write the expression of expansion work.
Here, gas constant is R, specific volume at state 2 and 3 is
Write the expression of back work ratio using the equations (I) and (II).
Here, temperature at state 1, 2, and 3 are
Conclusion:
Process 1-2: Isentropic
Calculate the ratio of
Here, pressure at state 1 and 2 is
Process 2-3: Constant pressure
Calculate the expression for
Here, volume at state 1 and 2 is
Process 3-1: Constant volume
Calculate the expression for
Substitute
Thus, the expression for the back work ratio as a function of k and r is
(c)
The expression for the cycle thermal efficiency as a function of k and r.
(c)
Answer to Problem 180RP
The expression for the cycle thermal efficiency as a function of k and r is
Explanation of Solution
Express out the heat addition and heat rejection in the process using first law to the closed system for processes 2-3 and 3-1.
Here, constant pressure specific heat is
Express the cycle thermal efficiency.
Conclusion:
Substitute
Substitute
Thus, the expression for the cycle thermal efficiency as a function of k and r is
(d)
The value of the back work ratio and thermal efficiency as r goes to unity.
(d)
Answer to Problem 180RP
The value of the back work ratio and thermal efficiency as r goes to unity is
Explanation of Solution
Recall the expression of back work ratio and apply the limits as r goes to infinity.
Thus, the value of the back work ratio as r goes to unity is
Recall the expression of cycle thermal efficiency and apply the limits as r goes to infinity.
Thus, the value of the thermal efficiency as r goes to unity is
From the results of cycle thermal efficiency and back work ratio values of 0 and 1, it shows that no expansion and net work can be done whether you add heat to the system when there is no compression
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- An air-standard cycle with variable specific heats is executed in a closed system with 0.0045 kg of air and consists of the following three processes: 1–2 v = Constant heat addition from 95 kPa and 17°C to 380 kPa 2–3 Isentropic expansion to 95 kPa 3–1 P = Constant heat rejection to initial state Use data from tables. Calculate the thermal efficiency % ? Hint : The answer should be a percentagearrow_forwardAir is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet temperature of 1000 K. Determine the required mass flow rate of air for a net power output of 70 MW, assuming both the compressor and the turbine have an isentropic efficiency of 85 percent. Assume constant specific heats at room temperature.arrow_forwardAn air-standard cycle is executed in a closed system with 0.5 kg of air and consists of the following three processes: 1-2 Isentropic compression from 100 kPa and 27C to 1 MPa 2-3 P = constant heat addition in the amount of 416 kJ 3-1 = 5 c1v + c2 heat rejection to initial state (c1 and c2 are constants) (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the heat rejected. (c) Determine the thermal efficiency. Assume constant specific heats at room temperature.arrow_forward
- An air-standard Carnot cycle is executed in a closed system between the temperature limits of 350 and 1200 K. The pressures before and after the isothermal compression are 150 and 300 kPa, respectively. If the net work output per cycle is 0.5 kJ, determine (a) the maximum pressure in the cycle, (b) the heat transfer to air, and (c) the mass of air. Assume variable specific heats for air.arrow_forwardA Brayton cycle with an air-powered regenerator has a pressure ratio of 8. The lowest and highest temperatures of the cycle are 310 K and 1150 K. The adiabatic efficiencies of the compressor and turbine are 75% and 82%, respectively, and the efficiency of the regenerator is 65%. Show the cycle in the T-s diagram. Consider the variation of specific heats with temperature. a) the temperature of the air at the exit of the turbine, b) the working of the cycle, c) Calculate the thermal efficiency of the cycle.arrow_forwardQ1: An ideal Otto cycle has a compression ratio of 8. At the beginning of the compression process, air is at 105 kPa and 28 °C, and 850 kJ/kg of heat is transferred to air during the constant-volume heat-addition process. Taking into account the variation of specific heats with temperature, determine (i) the pressure and temperature at the end of the heat addition process, (ii) the net work output, (iii) the thermal efficiency, and (iv) the mean effective pressure for the cycle.arrow_forward
- An air-standard cycle with variable specific heats is executed in a closed system with 0.003 kg of air, and it consists of the following three processes: 1-2 Isentropic compression from 3-1 v = constant heat rejection to initial state (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the maximum temperature in the cycle. (c) Determine the thermal efficiency.arrow_forwardAir is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet temperature of 1000 K. Determine the required mass flow rate of air for a net power output of 70 MW, assuming both the compressor and the turbine have an isentropic efficiency of 100 percent.arrow_forwardConsider an air-standard Otto cycle that has a compression ratio of 9.0 and a heat addition of 870 Btu/lbm. If the pressure and temperature at the beginning of the compression process are 14.0 psia and 40 °F, determine a. the maximum pressure and temperature for the cycle, b. the thermal efficiency, and c. the mean effective pressure, in psiaarrow_forward
- The pressure and temperature at the beginning of compression of an air-standard Diesel cycle are 90 kPa and 300 K, respectively. At the end of the heat addition, the pressure is 6821 kPa and the temperature is 1950 K. Determine the thermal efficiency of the cycle in %.arrow_forwardAn air-standard cycle with variable specific heats isexecuted in a closed system and is composed of the followingfour processes:1-2 v = constant heat addition from 14.7 psia and 80F inthe amount of 300 Btu/lbm2-3 P = constant heat addition to 3200 R3-4 Isentropic expansion to 14.7 psia4-1 P = constant heat rejection to initial state(a) Show the cycle on P-v and T-s diagrams.(b) Calculate the total heat input per unit mass.(c) Determine the thermal efficiency.arrow_forwardAn air standard cycle is executed in a closed system and composed of the following processes 1-2 Isentropic compression from 90 kPa and 77°C to 1 MPa 2-3 Pressure=constant heat addition in amount of 3500 kJ/kg 3-4 volume=constant heat rejection to 90 kPa 4-1 Pressure=constant heat rejection to initial state Calculate the maximum temperature in the cycle in kelvin and the cycle thermal efficiency?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





