
Concept explainers
(a)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority
The main chain or ring containing the highest-priority functional group is to be determined. The next step is to number the main chain or ring such that carbon atoms involving highest-priority functional group receive the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’... etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined, and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule, considering the given stereochemistry, is
Explanation of Solution
The given molecule is:
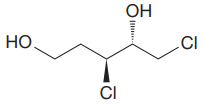
The molecule above contains two
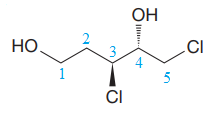
The corresponding locator numbers are also added before the prefix ‘di’. Thus, the name of the root is
There are two chiral centers in the given molecule at C3 and C4 carbon atoms.
The C3 carbon attached to Chlorine atom by a wedge bond has the S configuration, while the C4 carbon attached to the
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
(b)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority functional groups, the rules are slightly different. In the IUPAC name of such molecules, the final ‘e’ of ‘ane’, ‘ene’, or ‘yne’ is not removed in the suffix. Prefixes such as ‘di’, ‘tri, etc are written immediately before the suffix to specify the number of highest-priority functional groups. Add a locator number for each of the highest-priority functional groups immediately before the prefixes.
The main chain or ring containing the highest-priority functional group is to be determined. The next step is to number the main chain or ring such that carbon atoms involving the highest-priority functional group receive the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’… etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater atomic number has higher priority.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom, and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule is
Explanation of Solution
The given molecule is:
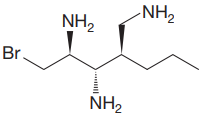
The molecule above contains three
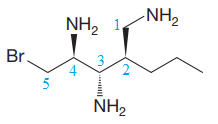
The corresponding locator numbers are also added before the prefix ‘tri’. Thus, the name of the root is
The carbon atoms C2 and C3 have two substituents attached.
One propyl group is attached to the C2 carbon atom of the root, while one bromine atom is attached to the C5 carbon atom of the root. The substituents are named in alphabetical order.
Thus, the complete IUPAC name for the compound without considering the stereochemistry is
There are three chiral centers in the given molecule.
The C2 carbon atom has R configuration.
The C3 carbon attached to
The C4 carbon attached to
Thus, the IUPAC name of the compound, considering the given stereochemistry is,
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
(c)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority functional groups, the rules are slightly different. In the IUPAC name of such molecules, the final ‘e’ of ‘ane’, ‘ene’, or ‘yne’ is not removed in the suffix. Prefixes such as ‘di’, ‘tri, etc are written immediately before the suffix to specify the number of highest-priority functional groups. Add a locator number for each of the highest-priority functional groups immediately before the prefixes.
The main chain or ring is to be determined to be the one that contains the highest-priority functional groups. The next step is to number the main chain or ring such that carbon atoms involving highest-priority functional group receives the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’… etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater atomic number has higher priority.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule is
Explanation of Solution
The given molecule is:
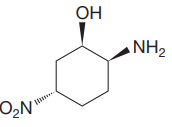
The molecule above contains one
The numbering is shown below:
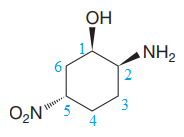
The name of the root is
There is one
Thus, the complete IUPAC name without considering the stereochemistry for the compound is
There are three chiral centers in the molecule.
The C1 carbon atom attached to the
The C2 carbon attached to the
The C5 carbon attached to the
Thus, the IUPAC name of the compound considering the given stereochemistry is
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
Want to see more full solutions like this?
Chapter E Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





