
Concept explainers
(a)
Interpretation:
The structure from the given IUPAC name with appropriate stereochemistry is to be drawn.
Concept introduction:
The root name in the given IUPAC name suggests the main chain or ring of carbon atoms in the compound. The suffix to root name indicates the highest priority functional group. The prefix with locant number indicates the number of substituents and their respective position at the main chain or a ring of carbon atoms.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to the chiral center on the basis of
Answer to Problem E.48P
The structure for IUPAC name
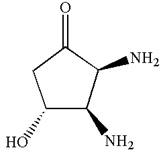
Explanation of Solution
The given IUPAC name is
The root name in this IUPAC name ‘cyclopentan’ represents the main ring of five carbon atoms. The suffix ‘one’ represents the highest priority functional group -
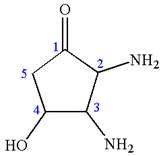
The structure has three chiral centers,
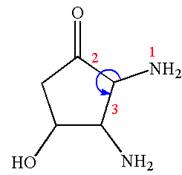
Thus, for absolute configuration
The absolute configuration at
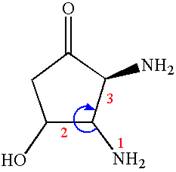
Thus, for absolute configuration
The absolute configuration at
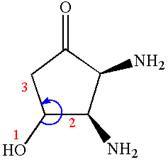
Thus for absolute configuration
Hence, the structure for is
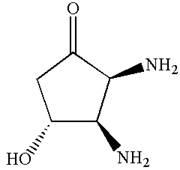
The structure for the given IUPAC name is drawn by identifying the highest priority functional group from the suffix, main chain from root name, and the position for the substituents from the locant with appropriate stereochemistry.
(b)
Interpretation:
The structure from the given IUPAC name with appropriate stereochemistry is to be drawn.
Concept introduction:
The root name in the given IUPAC name suggests the main chain or ring of carbon atoms in the compound. The suffix to root name indicates the highest priority functional group. The prefix with locant number indicates the number of substituents and their respective position at the main chain or at a ring of carbon atoms.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to chiral center on the basis of atomic number of directly bonded atom. If the sequence of priority order
Answer to Problem E.48P
The structure for IUPAC name
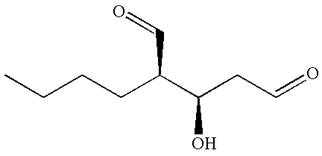
Explanation of Solution
The given IUPAC name is
The root name in this IUPAC name ‘pentane’ represents the main chain of five carbon atoms. The suffix ‘dial’ represents the highest priority functional group
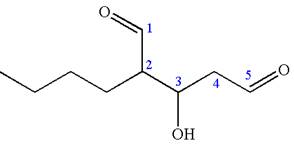
The structure has two chiral centers,
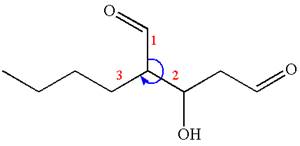
Thus, for the absolute configuration
The absolute configuration at
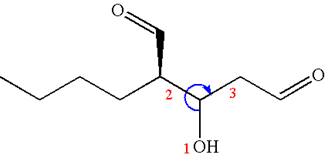
Thus for the absolute configuration
Hence, the structure for
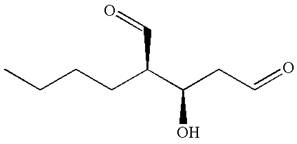
The structure for the given IUPAC name is drawn by identifying the highest priority functional group from the suffix, main chain from root name, and the position for the substituents from the locant with appropriate stereochemistry.
(c)
Interpretation:
The structure from the given IUPAC name with appropriate stereochemistry is to be drawn.
Concept introduction:
The root name in the given IUPAC name suggests the main chain or ring of carbon atoms in the compound. The suffix to root name indicates the highest priority functional group. The prefix with locant number indicates the number of substituents and their respective position at the main chain or at a ring of carbon atoms.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to chiral center on the basis of atomic number of directly bonded atom. If the sequence of priority order
Answer to Problem E.48P
The structure for IUPAC name
Explanation of Solution
The given IUPAC name is
The root name in this IUPAC name ‘heptane’ represents the main chain of seven carbon atoms. The suffix ‘
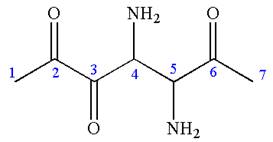
The structure has two chiral centers,
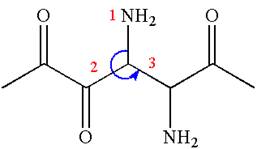
Thus, for the absolute configuration
The absolute configuration at
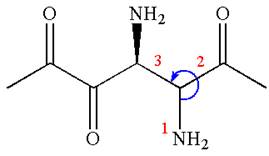
Thus, for the absolute configuration
Hence, the structure for

The structure for the given IUPAC name is drawn by identifying the highest priority functional group from the suffix, main chain from root name and the position for the substituents from the locant with appropriate stereochemistry.
(d)
Interpretation:
The structure from the given IUPAC name with appropriate stereochemistry is to be drawn.
Concept introduction:
The root name in the given IUPAC name suggests the main chain or ring of carbon atoms in the compound. The suffix to root name indicates the highest priority functional group. The prefix with locant number indicates the number of substituents and their respective position at the main chain or at a ring of carbon atoms.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to chiral center on the basis of atomic number of directly bonded atom. If the sequence of priority order
Answer to Problem E.48P
The structure for IUPAC name
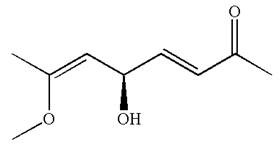
Explanation of Solution
The given IUPAC name is
The root name in this IUPAC name ‘octa’ represents the main chain of eight carbon atoms. The suffix ‘
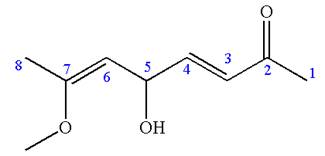
The structure has one chiral center,
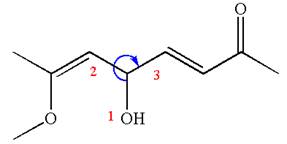
Thus for the absolute configuration
Hence, the structure for
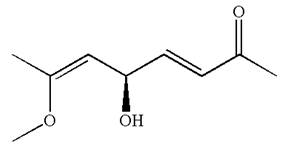
The structure for the given IUPAC name is drawn by identifying the highest priority functional group from the suffix, main chain from root name and the position for the substituents from the locant with appropriate stereochemistry.
Want to see more full solutions like this?
Chapter E Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Give the series of reactions below, identify and give the iupac name for the following compounds, in short identify and name A,B,Carrow_forwardDraw two diasteriomers of (1Z,4R)-1,4-dimethylcyclodecene and give the IUPAC name including E/Z and R/S notation.arrow_forward5-Hydroxyhexanal forms a six-membered cyclic hemiacetal, which predominates at equilibrium in aqueous solution. (a) Draw a structural formula for this cyclic hemiacetal. (b) How many stereoisomers are possible for 5-hydroxyhexanal? (c) How many stereoisomers are possible for this cyclic hemiacetal? (d) Draw alternative chair conformations for each stereoisomer and label groups axial or Also predict which of the alternative chair conformations for each stereoisomer is more stable.arrow_forward
- 2,3-Dimethylfumaric acid has a molecular formula C6H8O4. It undergoes oxidativecleavage to form two identical compound N. Compound N is then reacted withethylmagnesium bromide to form compound O. Compound O is then hydrolysed inacidic condition to form compound P. Draw the structure of compound N, O and P. PLEASE PROVIDE CLEAR HANDWRITING, and explantionarrow_forwardDraw a line structure for (4E,7S)-7-benzyl-5-fluoronon-4-en-2-one with threedimensional information where appropriate. Place the ketone group on the right-hand side of your drawing.arrow_forwardAssign R and S to all of the asymmetric centers and give the proper IUPAC name for the following compound.arrow_forward
- Below are two potential methods for preparing the same ether (Option A and B), but only one of them is actually successful. Identify the successful approach (A or B)arrow_forwardWrite out all the isomers of the compound with molecular formula C4H10O Select the normal/ primaryisomer and treat it with conc. H2SO4 and heat. Identify the reaction and give the product ‘A’ from it. Treatment of ‘A’ with HCl/H2O gives ‘B’ Treatment of ‘A’ with cold KMnO4/ -OH gives ‘C’ Treatment of ‘A’ with Hot KMnO4/-OH gives ‘D’ followed by acidification of the mixture to give ‘E’arrow_forwardA difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired configuration. a. Label this stereogenic center as R or S. b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship. c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forward
- A chiral alkyne A with molecular formula C6H10 is reduced with H2 and Lindlar catalyst to B having the R conguration at its stereogenic center. What are the structures of A and B?arrow_forwardPlants extract are widely used in many parts of Cameroon to treat infectious disease or related symptoms including abdominal pains, itching, urinary and respiratory ailments, fever, coughing and diarrhea. Harunmadagascarin C as depicted below is an extract from the plant Harungana madagascariensis that has been studied for potential antimicrobial activity. Which statement best describes the stereochemistry of the compound? a. Harunmadagascarin C is achiral and does not have any stereogenic or chiral centers. b. The two stereogenic centers are currently in the E configuration, and the double bond in the ring structure is not stereogenic. c. There are four possible stereoisomers for Harunmadagascarin C since, as depicted, there are 2 stereocenters in the compound. d. There is one chiral center present in the molecule.arrow_forwardTreatment of compound A (C8H17Br) with NaOCH2CH3 affords two constitutional isomers B and C. Ozonolysis of B affords CH2=O and (CH3CH2CH2)2C=O. Ozonolysis of C affords CH3CH2CH2COCH3 and CH3CH2CHO. What is the structure of A?arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

