
Concept explainers
(a)
Interpretation:
The IUPAC name for the given compound with appropriate stereochemical designation is to be assigned.
Concept introduction:
In naming organic compounds, the
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to the chiral center on the basis of
Answer to Problem E.46P
The name of the given compound is
Explanation of Solution
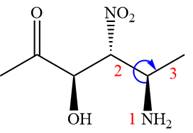
The given compound is:
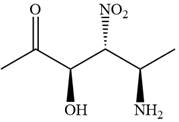
In this compound, the highest priority functional group is
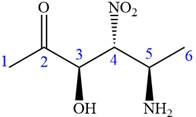
The ketonic carbon is numbered as
The fourth group, attached to chiral center
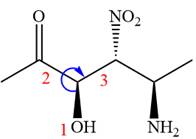
In the order of decreasing sequence rule
The fourth group attached to the chiral center
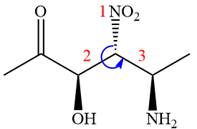
In the order of decreasing sequence rule,
The fourth group, attached to chiral center
In the order of decreasing sequence rule
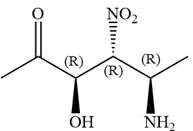
Hence the given compound is named as:
The given compound is named by identifying the main chain containing the functional group and the substituents attached with appropriate stereochemistry.
(b)
Interpretation:
The IUPAC name for the given compound with appropriate stereochemical designation is to be assigned.
Concept introduction:
In naming organic compounds, the functional groups other than highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the “e” from the normal ‘ane’, ‘ene’, or ‘yne’ ending and add the suffix that corresponds to the highest-priority functional group. Prefixes are used to denote number of identical substituents. Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of functional group and substituents on parent chain or ring is indicated by the respective locant number just before the suffix. The substituents are written in alphabetical order when writing the IUPAC name.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to chiral center on the basis of atomic number of directly bonded atom. If the sequence of priority order
Answer to Problem E.46P
The name of the given compound is:
Explanation of Solution
The given compound is:
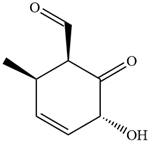
In this compound, the main ring is of six carbon atoms containing
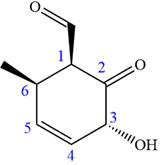
The ketonic carbon is numbered
The fourth group attached to the chiral center
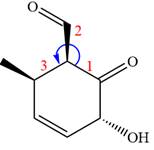
In the order of decreasing sequence rule
The fourth group attached to chiral center
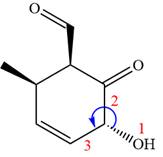
In the order of decreasing sequence rule
The fourth group attached to chiral center
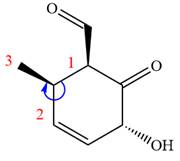
In the order of decreasing sequence rule
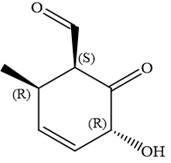
Hence, the given compound is named as:
The given compound is named by identifying the main chain containing functional group and the substituents attached with appropriate stereochemistry.
(c)
Interpretation:
The IUPAC name for the given compound with appropriate stereochemical designation is to be assigned.
Concept introduction:
In naming organic compounds, the functional groups other than highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the “e” from the normal ‘ane’, ‘ene’, or ‘yne’ ending and add the suffix that corresponds to the highest-priority functional group. Prefixes are used to denote number of identical substituents. Number the carbon chain in a way that the functional group and the substituents attached gets lowest number. The position of functional group and substituents on parent chain or ring is indicated by the respective locant number just before the suffix. The substituents are written in alphabetical order when writing the IUPAC name.
The stereochemical designation and the locators are enclosed in parenthesis at the very beginning of the name. The stereochemistry at the chiral center is determined by assigning the priorities to the groups attached to chiral center on the basis of atomic number of directly bonded atom. If the sequence of priority order
Answer to Problem E.46P
The name of given compound is:
Explanation of Solution
The given compound is:
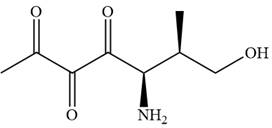
In this compound, the main chain is of seven carbon atoms which indicates the root name as ‘heptane’. The highest priority functional group is ketone. The chain has three carbonyl groups, therefore, the suffix ‘trione’ is added to the root name. The substituents are
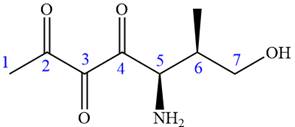
The ketonic carbons are numbered as
The fourth group attached to chiral center
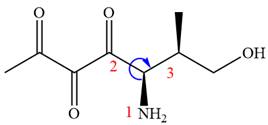
In the order of decreasing sequence rule
The fourth group attached to chiral center
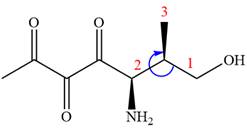
In the order of decreasing sequence rule
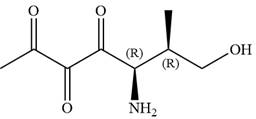
Hence, the given compound is named as:
The given compound is named by identifying the main chain containing functional group and the substituents attached with appropriate stereochemistry.
Want to see more full solutions like this?
Chapter E Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





