
Concept explainers
(a)
Interpretation :
Shape of ammonia molecule must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Ammonia molecule has total 4 atoms in which N is the central atom.
(a)
Answer to Problem C7.2RE
Shape of ammonia molecule is trigonal pyramidal.
Explanation of Solution
The structure of ammonia molecule is represented as follows:

N has total 4 pairs of electrons in the valence shell.
There are three bond pairs and lone pairs of electrons. N atom is sp3 hybridized and thus NH3 has trigonal pyramidal shape due to presence of one lone pair electrons.
b)
Interpretation :
Shape of silicon tetrachloride must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Silicon tetrachloride molecule has total 5 atoms in which Si is the central atom.
b)
Answer to Problem C7.2RE
Shape of silicon tetrachloride molecule is tetrahedral.
Explanation of Solution
The structure is represented as follows:
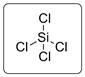
Si has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. Si atom is sp3 hybridized and thus SiCl4 has tetrahedral shape.
c)
Interpretation :
Shape of hydrogen sulfide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen sulfide molecule has total 3 atoms in which S is the central atom.
c)
Answer to Problem C7.2RE
Shape of hydrogen molecule is angular.
Explanation of Solution
The structure of H2S is represented as follows:
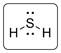
S has total 4 pairs of electrons in the valence shell.
There are two bond pairs and two lone pairs of electrons. S atom is sp3 hybridized and thus H2S has angular shape.
d)
Interpretation :
Shape of hydrogen cyanide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen cyanide molecule has total 3 atoms in which C is the central atom.
d)
Answer to Problem C7.2RE
Shape of hydrogen cyanide is linear.
Explanation of Solution
The structure is represented as follows:
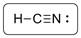
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is triple bonded with N and carbon is sp hybridized and thus HCN has linear shape.
e)
Interpretation :
Shape of formaldehyde must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Formaldehyde molecule has total 4 atoms in which C is the central atom.
e)
Answer to Problem C7.2RE
Shape of formaldehyde is trigonal planar.
Explanation of Solution
The structure of formaldehyde is represented as follows:
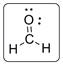
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is double bonded with O and single bonded with two hydrogen atoms. Carbon is sp2 hybridized and thus CH2N has trigonal planar shape.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry For Changing Times (14th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: The Central Science (13th Edition)
Chemistry (7th Edition)
Chemistry: A Molecular Approach
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





