
Concept explainers
Interpretation:
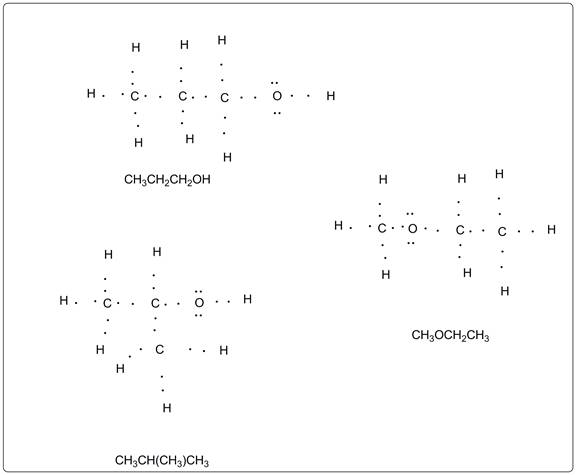
Octet rule must be used to draw Lewis structures of all the possible compound with the formula C3H8O.
Concept introduction:
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
C3H8O is a general molecular formula which represents structures for alcohols and ether.
Explanation of Solution
Two alcohols are possible with the general formula C3H8O. One is Propan-1-ol and another is propan-2-ol. In all the structures octet rule is followed as per which there are eight electrons in all the participating atoms including bond pairs and lone pairs of electrons except hydrogen.
One ether is possible with this formula which is methoxyethane with the formula CH3OCH2CH3.
All the Lewis dot structures possible for C3H8O are given below.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





