
Interpretation:
The equilibrium concentrations of the starting substances and products needs to be determined.
Concept introduction:
At equilibrium, the rate of formation of product is same as rate of deformation of reactant. Thus, the rate of decrease in the concentration of reactant is equal to the rate of increase in the concentration of product. At this point, there is no change in the concentration of reactant and product takes place.
Answer to Problem 12STP
The correct option is (A).
Explanation of Solution
The given equilibrium reaction is as follows:
At equilibrium, the rate of change of concentration of product and reactant will be same. When no change in the concentration takes place with time, it is represented by line parallel to time axis. It is after 25 second in the graph.
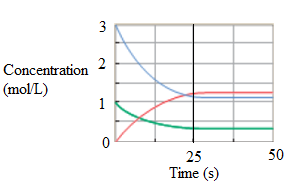
Now, at 25 sec, the concentration of reactant
Therefore, the correct option is (A).
Chapter U6 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties
Chemistry & Chemical Reactivity
Chemistry
Chemistry: A Molecular Approach
General Chemistry: Principles and Modern Applications (11th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





