
(a)
Interpretation :
Reactant and product graphs must be labeled.
Concept Introduction :
Concentration of reactants gradually decreases and that of product increases.
(a)
Answer to Problem 5E
Blue and green graphs are for reactants. Red graph is for product.
Explanation of Solution
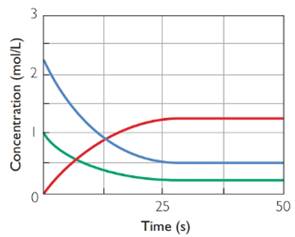
As per the graphs it is clear that with time blue and green graphs are coming downward, i.e., concentration decreases. Red graph is going upward, i.e., concentration is increasing. Thus blue and green graphs are for reactants and red graph is for product.
b)
Interpretation :
Equilibrium concentrations of reactant and product must be read from the graph.
Concept Introduction :
At equilibrium
b)
Answer to Problem 5E
Equilibrium concentrations of blue graph and green graph reactants are 0.5 M and 0.25 M respectively. equilibrium concentration of product (red graph) is 1.25 M.
Explanation of Solution
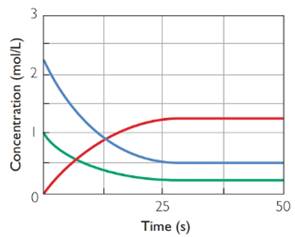
Equilibrium concentration is the concentration where the graphs are parallel to x-axis(time). At this point there is no change in concentration as the forward and backward reactions are taking place in same rate. From the graphs all the concentrations are found.
c)
Interpretation :
The time taken to reach equilibrium must be found out.
Concept Introduction :
Time when the forward reaction and backward reaction is taking place in same speed then equilibrium is attained.
c)
Answer to Problem 5E
It took around 25 s to reach equilibrium.
Explanation of Solution
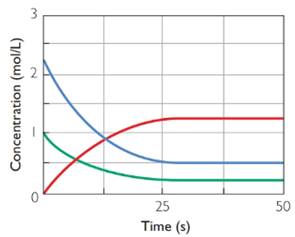
From the graphs it is clear that all the three graphs become parallel after 25 s. At this time point forward and backward reaction is taking place in same speed. So it took 25 s for the attainment of equilibrium.
d)
Interpretation :
The variable which is equal at equilibrium must be explained.
Concept Introduction :
At equilibrium rate of forward and backward reactions are same.
d)
Answer to Problem 5E
At equilibrium rate of forward reaction is “equal” to rate of backward reaction.
Explanation of Solution
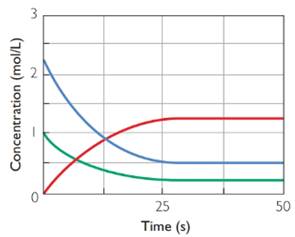
After 25 s the reaction has attained equilibrium. At this point all the three graphs are parallel to time axis which means there is no change in concentration of reactants or product with time. So rate of forward reaction is “equal” to rate of backward reaction at equilibrium.
Chapter U6 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
CHEMISTRY-TEXT
General Chemistry: Atoms First
Chemistry: The Central Science (14th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





