
Interpretation:
The time taken for the given reaction to reach at equilibrium needs to be determined.
Concept introduction:
Answer to Problem 14STP
Option (C) is correct option.
Explanation of Solution
Reason for correct option:
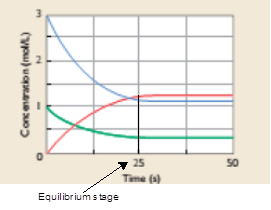
In the rerversible reaction, reaction moves in both direction and reaches at equilibrium position. At equilibrium position, the rate of forward reaction is equal to rate of backward direction. In other words, the rate of consumption of reactant equals to rate of formation of product. According to given graph, at 25 sec, the rate of consmption of reactant equals to rate of formation of product, thus, reaction must be at equilbrium at 25 sec.
Reasons for incorrect options:
After 25 sec, the rate of consmption of reactant equals to rate of formation of product therefore reaction must be at equilibrium at 25 sec.
Chapter U6 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry: Matter and Change
Chemistry: Structure and Properties
Organic Chemistry
Chemistry: The Central Science (13th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Organic Chemistry (8th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





