13C NMR Spectrum (50.0 MHz. CDCI, solution) 3500-2500 IR Spectrum (liquid film) 1715 proton decoupled solvent 4000 3000 2000 1600 1200 800 V (cm') 200 O 8 (ppm) 160 120 80 40 아 Mass Spectrum 1H NMR Spectrum (200 MHz, CDCI, solution) 45 M** 74 57 C3H6O2 40 80 120 160 200 240 280 12 11 TMS m/e No significant UV 8 7 6 4 1 8 (ppm) 10 9 absorption above 220 nm % of base peak
13C NMR Spectrum (50.0 MHz. CDCI, solution) 3500-2500 IR Spectrum (liquid film) 1715 proton decoupled solvent 4000 3000 2000 1600 1200 800 V (cm') 200 O 8 (ppm) 160 120 80 40 아 Mass Spectrum 1H NMR Spectrum (200 MHz, CDCI, solution) 45 M** 74 57 C3H6O2 40 80 120 160 200 240 280 12 11 TMS m/e No significant UV 8 7 6 4 1 8 (ppm) 10 9 absorption above 220 nm % of base peak
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.11QAP
Related questions
Question
100%
Analyse the spectra and write the name of the compound. Also write brief explanation for each spectra verifying the parts of your suggested molecule (For IR indicate the
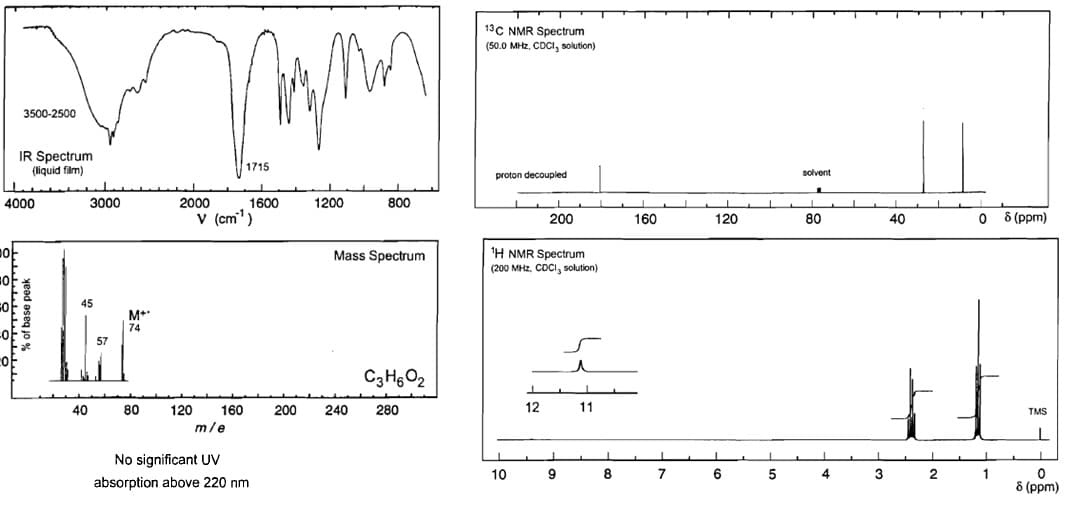
Transcribed Image Text:13C NMR Spectrum
(50.0 MHz. CDCI, solution)
3500-2500
IR Spectrum
(liquid film)
1715
proton decoupled
solvent
4000
3000
2000
1600
1200
800
V (cm')
80
O 8 (ppm)
200
160
120
40
아
Mass Spectrum
1H NMR Spectrum
(200 MHz, CDCI, solution)
45
M**
74
57
C3H6O2
40
80
120
160
200
240
280
12
11
TMS
m/e
No significant UV
10
9
8
7
6
absorption above 220 nm
1
8 (ppm)
% of base peak
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
