5 Two metal blocks of equal mass M of the same sub- stance, one at an initial temperature Ti and the other at an initial temperature T2, are placed in a well-insulated (adiabatic) box of constant volume. A device that can produce work from a flow of heat across a tempera- ture difference (i.., a heat engine) is connected between the two blocks. Develop expressions for the maximum amount of work that can be obtained from this process and the common final temperature of the blocks when this amount of work is obtained. You may assume that 14 Chapter 4: Entropy: An Additio the heat capacity of the blocks does not vary with temperature. 24 4 an d4.5-
5 Two metal blocks of equal mass M of the same sub- stance, one at an initial temperature Ti and the other at an initial temperature T2, are placed in a well-insulated (adiabatic) box of constant volume. A device that can produce work from a flow of heat across a tempera- ture difference (i.., a heat engine) is connected between the two blocks. Develop expressions for the maximum amount of work that can be obtained from this process and the common final temperature of the blocks when this amount of work is obtained. You may assume that 14 Chapter 4: Entropy: An Additio the heat capacity of the blocks does not vary with temperature. 24 4 an d4.5-
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
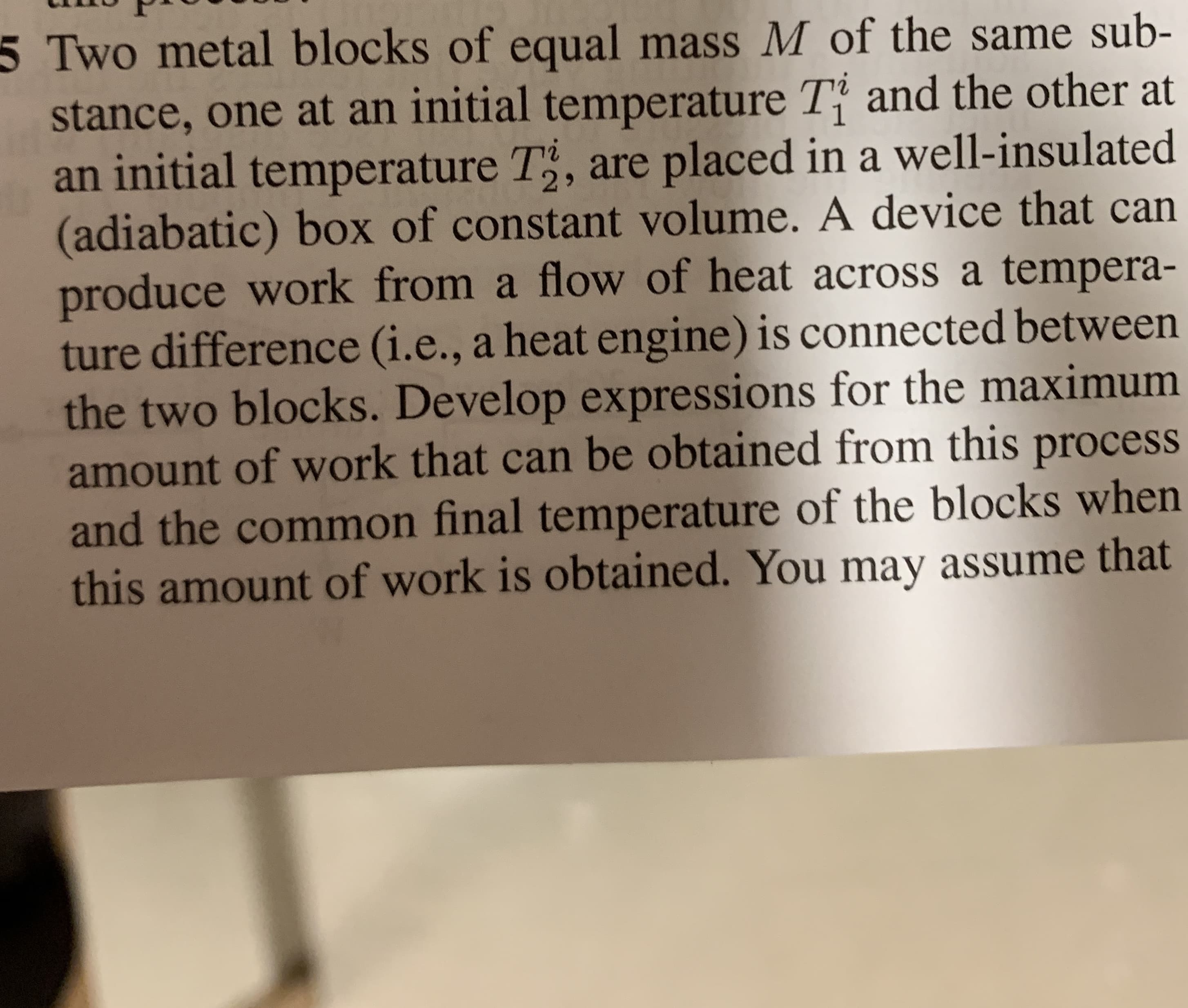
Transcribed Image Text:5 Two metal blocks of equal mass M of the same sub-
stance, one at an initial temperature Ti and the other at
an initial temperature T2, are placed in a well-insulated
(adiabatic) box of constant volume. A device that can
produce work from a flow of heat across a tempera-
ture difference (i.., a heat engine) is connected between
the two blocks. Develop expressions for the maximum
amount of work that can be obtained from this process
and the common final temperature of the blocks when
this amount of work is obtained. You may assume that

Transcribed Image Text:14
Chapter 4: Entropy: An Additio
the heat capacity of the blocks does not vary with
temperature.
24 4 an
d4.5-
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 9 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The