
Consider an ideal steam regenerative Rankine cycle with two feedwater heaters, one closed and one open. Steam enters the turbine at 10 MPa and 600°C and exhausts to the condenser at 10 kPa. Steam is extracted from the turbine at 1.2 MPa for the closed feedwater heater and at 0.6 MPa for the open one. The feedwater is heated to the condensation temperature of the extracted steam in the closed feedwater heater. The extracted steam leaves the closed feedwater heater as a saturated liquid, which is subsequently throttled to the open feedwater heater. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the mass flow rate of steam through the boiler for a net power output of 400 MW and (b) the thermal efficiency of the cycle.
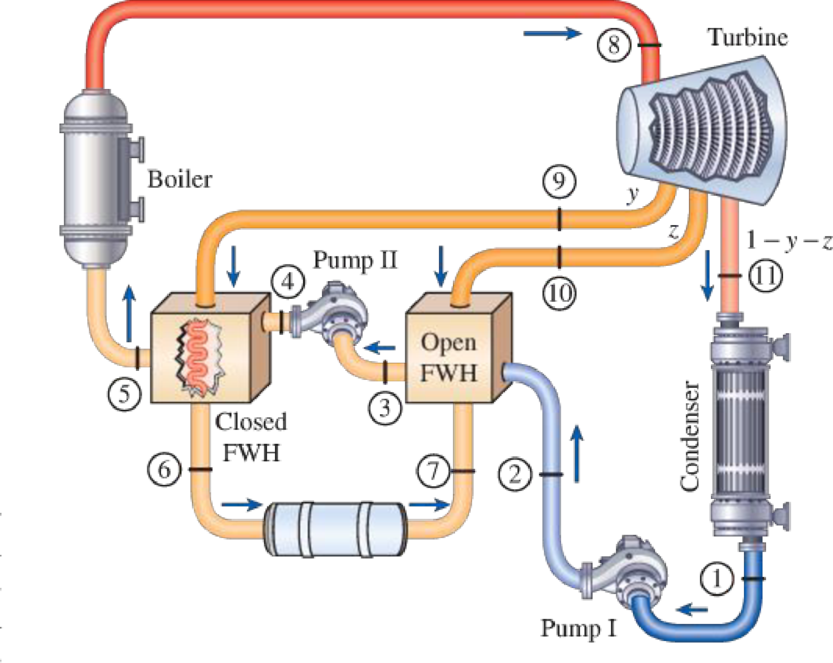
FIGURE P10–53
(a)
The mass flow rate of the steam through the boiler for a net power output of
Answer to Problem 53P
The mass flow rate of the steam through the boiler for a net power output of
Explanation of Solution
Draw the schematic diagram of the given ideal regenerative Rankine cycle as shown in
Figure 1.
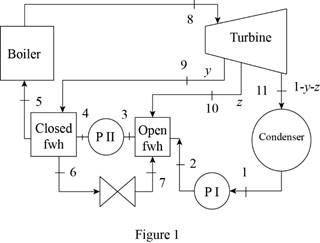
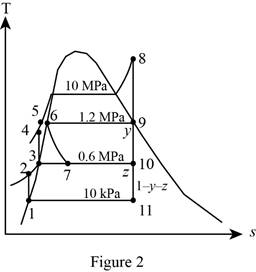
Draw the
Figure 2.
Here, water (steam) is the working fluid of the regenerative Rankine cycle. The cycle involves three pumps.
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
At state 11:
The steam expanded to the pressure of
The quality of water at state 11 is expressed as follows.
The enthalpy at state 11 is expressed as follows.
Here, the enthalpy is
Refer Figure 1 and 2.
Write the formula for heat in
Here, the mass fraction steam extracted from the turbine to the feed water entering the boiler via closed feed water heater
Write the general equation of energy balance equation.
Here, the rate of net energy inlet is
At steady state the rate of change of net energy of the system
Refer Equation (IX).
Consider the closed feed water heater alone.
Here,
Write the energy balance equation for closed feed water heater.
Rewrite the Equation (X) in terms of mass fraction
Refer Equation (IX).
Consider the open feed water heater alone.
Here,
Write the energy balance equation for open feed water heater.
Rewrite the Equation (XII) in terms of mass fraction
Write the formula for net work output of the cycle.
Write the formula for mass flow rate of the cycle.
At state 1: (Pump I inlet)
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 3: (Pump II inlet)
The water exits the open feed water heater-I as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 6: (boiler inlet or closed feed water exit)
The feed water is heated to the condensation temperature
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
The extracted steam exits the closed feed water heater as a saturated liquid at the pressure of
The enthalpy
At State 5:
The extracted steam exits the closed feed water heater as a saturated liquid at the temperature of
Refer Table A-4, “Saturated water-Temperature table”.
The enthalpy
At state 7:
The steam at state 6 is throttled to state 7. During throttling the enthalpy kept constant.
At state 8:
The steam enters the turbine as superheated vapor.
Refer Table A-6, “Superheated water”.
The enthalpy
From Figure 2,
At state 9:
The steam is extracted at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state 10:
The steam is extracted at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state 11:
The steam enters the condenser at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Conclusion:
Substitute
Substitute
Substitute
Equation (III).
Substitute
From Figure 1,
Substitute
Substitute
Equation (VI).
Consider the open feed water heater alone.
Substitute
Consider the closed feed water heater alone.
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the mass flow rate of the steam through the boiler for a net power output of
(b)
The thermal efficiency of the cycle.
Answer to Problem 53P
The thermal efficiency of the cycle is
Explanation of Solution
Write the formula for thermal efficiency of the cycle
Conclusion:
Substitute
Thus, the thermal efficiency of the cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics: An Engineering Approach
- 2. A steam power plant operates on the Rankine cycle in which the steam enters the turbine at 16 MPa and 600°C and the condensate leaves the condenser at 10 kPa. If the isentropic efficiency of the turbine is 87 percent and the isentropic efficiency of the pump is 85 percent, determine (a) the thermal efficiency of the cycle and (b) the net power output of the plant for a mass flow rate of 15 kg/s.arrow_forwardA steam power plant operates on an ideal regenerative Rankine cycle with two open feedwater heaters. Steam enters the turbine at 10 MPa and 600C and exhaust to the condenser at 5 kPa. Steam is extracted from the turbine at 0.6 and 0.2 MPa. Water leaves both feedwater heaters as a saturated liquid. The mass flow rate of steam through the boiler is 22 kg/sec. Show the cycle on a T-s diagram, and determine (a) Total power requirement of the pump in Hp, (b) Turbine power output in MW, (c) the net power output of the power plant in MW, (d) mass of steam extracted for feedwater heating in kg/sec,arrow_forwardHow does the thermal efficiency of an ideal Otto cycle change with the compression ratio of the engine and the specific heat ratio of the working fluid?arrow_forward
- Consider a steam power plant operating on the ideal Rankine cycle. Steam enters the turbine at 3 MPa and 350°C and is condensed in the condenser at a pressure of 10 kPa. Determine the thermal efficiency of this power plant, the thermal efficiency if steam is superheated to 600°C instead of 350°C, and the thermal efficiency if the boiler pressure is raised to 15 MPa while the turbine inlet temperature is maintained at 600°C. Draw the T-S diagram of each condition.arrow_forwardConsider a steam power plant that operates on a reheat Rankine cycle and has a net power output of 80 MW. Steam enters the high-pressure turbine at 10 MPa and 500°C and the low-pressure turbine at 1 MPa and 500°C. Steam leaves the condenser as a saturated liquid at a pressure of 10 kPa. The isentropic efficiency of the turbine is 80%, and that of the pump is 95 %. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a)the quality (or temperature, if superheated) of the steam at the turbine exit, (b)the thermal efficiency of the cycle, and (c)the mass flow rate of the steam.arrow_forwardA steam power plant operating on the intermediate steam Rankine cycle produces a net power of 160 MW. Water vapor enters the turbine at a pressure of 15 MPa and a temperature of 600 oC, and the condenser at a pressure of 15 kPa. The isentropic efficiency of the turbine is 85 percent and the isentropic efficiency of the pumps is 90 percent. In order to heat the feed water, some steam is separated from the turbine at a pressure of 0.6 MPa and sent to the open feedwater heater and exits the heater as a saturated liquid. Accordingly, fill in the blanks below. (Pump 1 inlet will be considered as saturated liquid.) a) The mass flow rate of the steam passing through the boiler is m5= ..... kg/s. b) The mass flow rate of the steam separated from the turbine to heat the feed water is m6= ...... kg/s. c) The heat entering the cycle is Qin = ...... kW. d) The heat released from the cycle is Qout = ...... kW. e) The power produced in the turbine is WTurbine= ...... kW. f) The power consumed in…arrow_forward
- The second steam power plant operates on a regenerative Rankine cycle, where water is used as the working fluid. Steam enters the turbine at 8 MPa and 450 ºC. After isentropic expansion in the first stage of the turbine, steam is extracted at an intermediate pressure of 0.75 MPa and passed to a closed feedwater heater. The feedwater leaves the heater at 8 MPa and a temperature equal to the saturation temperature at 0.75 MPa. The saturated liquid condensate from the feedwater heater leaves at 0.75 MPa and is pumped into the feedwater line. The condenser pressure is 7.5 kPa. The net power output from the cycle is 100 MW Task 41. Draw the TS diagram for this system and clearly label all the states2. Find the rate of heat transfer to the working fluid passing through the steam generator.3. Determine the thermal efficiency of the cycle.4. Explain how the turbine work output, heat supplied, heat rejected and moisture content at turbine exit change when regeneration is added to a simple ideal…arrow_forwardConsider a steam power plant that operates on an ideal regenerative Rankine cycle and has a net power output of 150 MW. Steam enters the turbine at 10 MPa and 500°C and the condenser at 15 kPa. Steam is extracted from the turbine at 0.5 MPa to heat the feedwater in an open feedwater heater. Water leaves the feedwater heater as a saturated liquid. Show the cycle on a T-s diagram, and determine (a) the mass flow rate of steam through the boiler, and (b) the thermal efficiency of the cycle.arrow_forward1. What is the Isentropic Work of the Steam using the Ideal Rankine Cycle when the system is set at 400 kPaa Boiler Pressure and 105 kPaa Condenser Pressure when the system is generating power at 9,500 seconds? 2. With the Turbine work output, what is the Isentropic efficiency of the Turbine from the above settings?arrow_forward
- Consider a 150-MW steam power plant that operates on a simple Rankine cycle. Steam enters the turbine at 7 MPa and 500°C and is cooled in the condenser at 10 kPa. Calculate the plant monthly consumption of coal if its heating value is 26,450 kJ/kg and the boiler has an efficiency of 90%. Assume an isentropic efficiency of 87% for both the turbine and the pump.arrow_forwardA steam power plant operates on an ideal Rankine cycle with two stages of reheat and has a net power output of 75 MW. Steam enters all three stages of the turbine at 550C. The maximum pressure in the cycle is 10 MPa, and the minimum pressure is 30 kPa. Steam is reheated at 4 MPa the first time and at 2 MPa the second time. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the thermal efficiency of the cycle, and (b) the mass flow rate of the steam.arrow_forwardA steam power plant operates on an ideal regenerative Rankine cycle with two open feedwater heaters. Steam enters the turbine at 8 MPa and 550C and exhausts to the condenser at 10 kPa. Steam is extracted from the turbine at 0.6 and 0.2 MPa. Water leaves both feedwater heaters as a saturated liquid. The mass flow rate of steam through the boiler is 16 kg/s. Show the cycle on a T-s diagram, and determine (a) the net power output of the power plant and (b) the thermal efficiency of the cycle.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





