
The gas-turbine portion of a combined gas–steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The combustion gases leaving the gas turbine are used to heat the steam to 400°C at 10 MPa in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. The steam leaving the turbine is condensed at 15 kPa. Assuming all the compression and expansion processes to be isentropic, determine (a) the mass flow rate of the steam, (b) the net power output, and (c) the thermal efficiency of the combined cycle. For air, assume constant specific heats at room temperature.
(a)
The mass flow rate of the steam.
Answer to Problem 82P
The mass flow rate of the steam is
Explanation of Solution
Show the
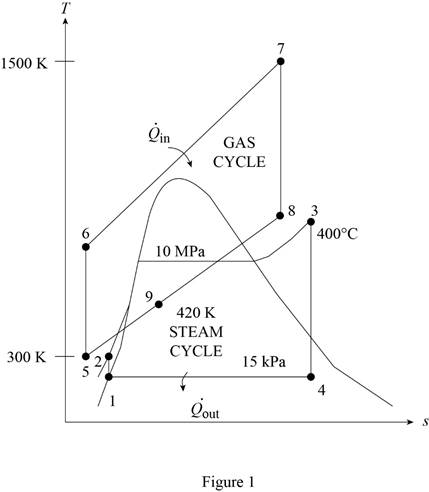
Determine the temperature of gas cycle at state 6.
Here, the temperature of gas cycle at state 5 is
Determine the rate of heat transfer into the gas turbine.
Here, the mass flow rate of air is
Determine the power rate for compressor of gas turbine.
Determine the temperature of gas cycle at state 8.
Here, the pressure of gas cycle at state 8 is
Determine the power rate for gas turbine of gas turbine.
Determine the net power output of the gas cycle.
Determine input work done per unit mass of the isentropic process for the steam cycle.
Here, the specific volume of the steam is
Determine the specific enthalpy at state 2 of the steam cycle.
Here, the specific enthalpy at the state 1 of the steam cycle is
Determine the quality at state 4 of the stream cycle.
Here, the specific entropy at state 4 is
Determine the specific enthalpy at state 4 of the steam cycle.
Here, the specific enthalpy of saturated liquid is
Write the expression for the steady-flow energy balance equation.
Here, the total energy rate of entering the system is
Substitute
Here, the temperature of gas cycle at state 8 is
Determine the power rate for gas turbine of steam cycle.
Here, the mass flow rate of the steam is
Determine the power rate of the isentropic process for the steam cycle.
Here, the mass flow rate of the steam is
Determine the net power output of the steam cycle.
Conclusion:
From the Table A-2, “Ideal-gas specific heats of various common gases”, obtain the value of specific heat of constant pressure and the ratio of specific heat at temperature of
Substitute 300 K for
Substitute
Substitute
Substitute 1500 K for
Substitute
Substitute 11547 kW for
From the Table A-4, “Saturated water-Pressure table”, obtain the value of the initial specific enthalpy at liquid state, specific volume at the liquid state, the specific entropy at liquid state, the specific enthalpy change upon vaporization at pressure, and the specific entropy change upon vaporization at pressure of 15 kPa as:
Substitute
Substitute
From the Table A-6, “Superheated water”, obtain the value of the specific enthalpy at state 3 and the specific entropy at state 3 at pressure of 10 MPa and temperature of
Substitute
Substitute 0.7528 for
Substitute
Thus, the mass flow rate of the steam is
Substitute
Substitute
Substitute 1384.013 kW for
(b)
The net work output of the combined cycle.
Answer to Problem 82P
The net work output of the combined cycle is
Explanation of Solution
Determine the net power output of combined cycle.
Conclusion:
Substitute 1371 kW for
Thus, the net work output of the combined cycle is
(c)
The thermal efficiency of the combined cycle.
Answer to Problem 82P
The thermal efficiency of the combined cycle is
Explanation of Solution
Determine the thermal efficiency of the combined cycle.
Conclusion:
Substitute 7819 kW for
Thus, the thermal efficiency of the combined cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics: An Engineering Approach
- Consider a regenerative gas-turbine power plant with two stages of compression and two stages of expansion. The overall pressure ratio of the cycle is 9. The air enters each stage of the compressor at 300 K and each stage of the turbine at 1200 K. Accounting for the variation of specific heats with temperature, determine the minimum mass flow rate of air needed to develop a net power output of 110 MW.arrow_forwardAir enters the compressor of a gas turbine at 100 kPa and 25°C. Determine the back work rate and thermal efficiency of the Brayton cycle for a pressure ratio of 5 and a maximum temperature of 850°C.arrow_forwardConsider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the quality of the steam at the turbine exit, (b) the thermal efficiency of the cycle, and (c) the mass flow rate of the steam.arrow_forward
- QII/ The gas-turbine portion of a combined gas—steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The combustion gases leaving the gas turbine are used to heat the steam to 400 0 C at 10 MPa in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. The steam leaving the turbine is condensed at 15 kPa. Assuming all the compression and expansion processes to be isentropic, determine (a) the mass flow rate of the steam, (b) the net power output, and (c) the thermal efficiency of the combined cycle. For air, assume constant specific heats at room temperature. Answers:(a) 1.275 kg/s, (b) 7819 kW, (c) 66.4 percent.arrow_forwardCONSIDER A STEAM POWER PLANT OPERATING ON THE IDEAL REHEAT RANKINE CYCLE. STEAM ENTERS THE HIGH-PRESSURE TURBINE AT 15 MPA AND 600 °C AND IS CONDENSED IN THE CONDENSER AT A PRESSURE OF 10 KPA. ASSUMING THE REHEAT TEMPERATURE IS ALSO 600 °C AND IF THE MOISTURE CONTENT OF THE STEAM AT THE EXIT OF THE LOW-PRESSURE TURBINE IS NOT TO EXCEED 10%, DETERMINE: THE PRESSURE AT WHICH STEAM SHOULD BE REHEATED,arrow_forwardIn a steam power plant that operates based on a Rankine cycle, the operating pressures of the steam generator and the condenser are 10,000 and 10 kPa, respectively. If the turbine inlet stream is saturated vapor and the condenser outlet flow is saturated liquid, determine the specific heat transfers in the steam generator and the condenser, the specific work involved in the turbine and the pump, and the thermal efficiency and the BWR of the cycle. Also, if the power plant produces 250 MW power, determine the mass flow rate of the cycle’s working fluid.arrow_forward
- In reheat cycle, the steam generator produces 10 MPa and 600 degree celsius. Steam is removed from the high-pressure turbine at pressure of 2 MPa and reheat to a temperature of 600 deg C. Saturated water exits the condenser of the cycle pressure at 20 KPa. If the mass flow rate of the cycle is 125 kg/sec, determine the thermal efficiency and the net power output of the cycle.arrow_forwardHow do the inefficiencies of the turbine and the compressor affect the thermal efficiency of a gas-turbine engine?arrow_forwardThe enthalpy of steam entering the turbine inlet is 2750 kJ/kg and leaving turbine at 1750 kJ/kg. The enthalpy of saturated liquid is 190 kJ/ kg and enthalpy of dry saturated steam 2600 kJ/kg corresponding to the condenser pressure. The quality of steam at the exit of turbine isarrow_forward
- Consider a steam power plant operating on the ideal Rankine cycle. Steam enters the turbine at 3 MPa and 350°C and is condensed in the condenser at a pressure of 10 kPa. Determine the thermal efficiency of this power plant, the thermal efficiency if steam is superheated to 600°C instead of 350°C, and the thermal efficiency if the boiler pressure is raised to 15 MPa while the turbine inlet temperature is maintained at 600°C. Draw the T-S diagram of each condition.arrow_forwardWhat components can be added to a simple open cycle gas turbine power plant in order to increase its efficiency? Given the gas turbine is powered with auto-diesel fuel.arrow_forwardR134a is used as a working fluid in a cooling system with a cooling capacity of 50 kJ/s operating according to the ideal vapor compression refrigeration cycle. The refrigerant enters the compressor as saturated steam at a pressure of 120 kPa and is compressed to a pressure of 650 kPa. The mass flow of R-134a is 0.10 kg/s. Calculate the efficiency coefficient of the cycle. A-6,896B-4.359C-5.417D-5.668E-4.987 (Note: This question is Turkish) orginal question: İdeal buhar sıkıştırmalı soğutma çevrimine göre çalışan soğutma kapasitesi 50 kJ/s olan bir soğutma sisteminde iş akışkanı olarak R134a kullanılmaktadır. Soğutucu akışkan kompresöre 120 kPa basınçta doymuş buhar olarak girmekte ve 650 kPa basınca sıkıştırılmaktadır. R-134a’nın kütlesi debisi 0.10 kg/s’dir. Çevrimin etkinlik katsayısını hesaplayınız.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





