
Concept explainers
Give the IUPAC name for each compound.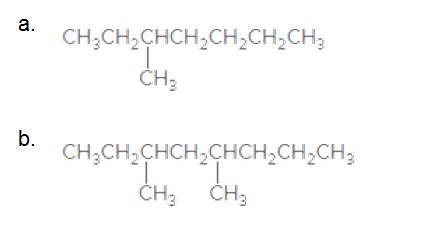
c.
e.
(a)
Interpretation:
The IUPAC nomenclature of alkanes having only one branched-chain needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3-Methylheptane
Explanation of Solution
The longest carbon chain has 7 carbon.
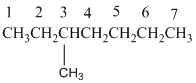
And there is a methyl group in the no 3 carbon. As the branch group has only one CH3, it is called 'Methyl'.
So, following by the above mention rule, the name of the alkane is 3 (the position of the branch) + Methyl (the name of the branch)+hept ( 7 carbon in main chain)+ "-ane"= 3-Methylheptane
(b)
Interpretation:
The IUPAC nomenclature of alkanes having 2 same brunches needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3,5-dimethyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
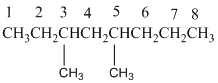
And there are two methyl groups in the no. 3 and no. 5 carbons. As the branch group has only one CH3, it is called 'Methyl'.
So, following by the above mention rule, the name of the alkane is 3,5 (the position of the branch) + dimethyl (the name of the branch, 'di' as two methyl are present)+ oct( 8 carbon in main chain)+ "-ane"= 3,5-dimethyloctane.
(c)
Interpretation:
The IUPAC nomenclature of Alkanes having 2 same brunches in same carbon needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3,3-diethylhexane
Explanation of Solution
The longest carbon chain has 8 carbon.
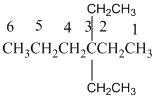
And there are two ethyl groups in the no. 3 carbon. As the branch groups have CH2CH3, it is called 'ethyl'.
So, following by the above mention rule, the name of the alkane is 3,3 (the position of the branch) + diethyl (the name of the branch, 'di' as two ethyl are present)+ hex( 6 carbon in main chain)+ "-ane"= 3,3-diethylhexane.
(d)
Interpretation:
The IUPAC nomenclature of Alkanes having skeletal structure needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
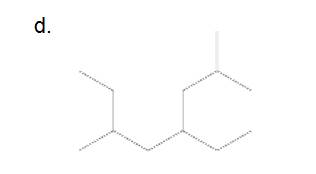
Answer to Problem 45P
4-ethyl-2,6-dimethyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
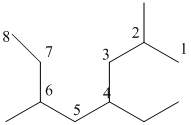
And there are two methyl groups in the no. 2 and 6 carbon, one ethyl group in no. 4carbon. As the branch groups have CH3and CH2CH3, they are called 'methyl' and 'ethyl' simultaneously.
So, following by the above mention rule, the name of the alkane is 4 (the position of the branch having alphabetically order 1st) +ethyl (the name of the branch) + 2,6 (position of 2nd brunch) + dimethyl( name of the brunch and 'di' as two methyl are present)+ oct ( 8 carbon in main chain)+ "-ane"= 4-ethyl-2,6-dimethyloctane
(e)
Interpretation:
The IUPAC nomenclature of alkanes having different brunches in different position needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
6-ethyl-2-methyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
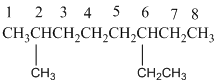
And there is one methyl group in the no. 2 carbon, one ethyl group in no. 6 carbon. As the branch groups have CH3and CH2CH3, they are called 'methyl' and 'ethyl' simultaneously.
So, following by the above mention rule, the name of the alkane is 6 (the position of the branch having alphabetically order 1st) + ethyl (the name of the branch) + 2 (position of 2nd brunch) + methyl( name of the brunch )+ oct ( 8 carbon in main chain)+ "-ane"= 6-ethyl-2-methyloctane
(f)
Interpretation:
The IUPAC nomenclature of Alkanes having lots of groups present in different position needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
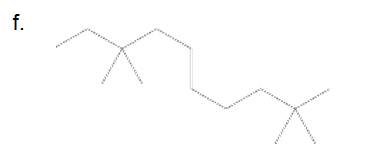
Answer to Problem 45P
3,3,9,9-tetramethylundecane
Explanation of Solution
The longest carbon chain has 8 carbon.
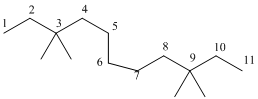
And there are four methyl groups in the no. 3 and 9 carbon. As the branch groups have CH3, it is called 'methyl'.
So, following by the above mention rule, the name of the alkane is 3,3,9,9 (the position of the branch) + tetramethyl ( name of the brunch and 'tetra' as four methyl are present)+ undacane ( 11 carbon in main chain)+ "-ane"= 3,3,9,9-tetramethylundecane.
Want to see more full solutions like this?
Chapter 12 Solutions
General, Organic, and Biological Chemistry - 4th edition
- the context is "Cosmone is a molecule used by fragrance manufacturers to provide a rich and elegant musky essence to many perfumes. Cosmone has the molecular formula C15H26O." I need help on part (h).arrow_forwardsample weight: KOH: 1g KOtBu 1g 1-propanol: 1g 1-butanol 0.742g calculate the thoeretical and percent yields of methylbutenesarrow_forwardWhich of the following molecules has only single bonds. A. CHCHCH3 B. CH2CHCH3 C. CH3CH2CCH D. CH3CH3 E. CH2CH2 Which of the following molecules has a carbon-to-carbon double bond? A. CH3CCH B. CHCH C. CH3CH3 D. CH3CH2CH3 E. CH2CHCH3arrow_forward
- 2,4-D is a synthetic auxin (a plant hormone) used in the control of broadleaf weeds, and one of the most widely used herbicides in the world. It is produced commercially by the following reaction: chloroacetic acid + dichlorophenol → hydrochloric acid + 2,4-D C2H3ClO2 + C6H4Cl2O → HCl + C8H6Cl2O3 How much chloroacetic acid does it take to manufacture 5.18 grams of 2,4-D?arrow_forwardBreak compound A into several small (less or equals to 6 carbon atom) molecules. You need to explain the reasons why you choose to break those bonds.arrow_forwardHOCI HF HCN H₂SO4 HOBr 2.29 x 10-8 O 2.30 K₂= 3.510-8 O 1.17 x 108 O 1.45 x 10-7 K₂ = 7.2 10-4 K₂ = 4.010-10 K₁ = very large K₂ = 1.2 10-2 Ka = 2.5210-⁹ (COOH)2 CH3COOH C6H5NH2 NH3 K₁ = 5.9 x 10-² K₂ = 6.4 x 10-5 K₂ = 1.8 x 10-5 Refer to Equilibrium Constants. What is the [H3O+] of a solution that is 0.0100 M in HOCland 0.0300 M in NaOCI? Kb = 4.2 x 10-10 Kb = 1.8 x 10-5arrow_forward
- Name the following organic compounds a) CH2 ═ CH – CH2 –CH2– CH2 – CH3 b) CH3CH2CH2COCH2CH3 c) CH3 – CH2 – CH2 – CH2 – C≡C – CH2-CH2-CH3 d) CH3 – CH2 – CH2 – CH2 – COO-CH2 – CH3 e) CH3 – CH2 – CH2 – CH2 – CH2 - O-CH2 – CH3arrow_forwardRank the following groups in order of decreasing priority. a.−COOH, −H, −NH2, −OH b.−H, −CH3, −Cl, −CH2Cl c. −CH2CH3, −CH3, −H, −CH(CH3)2 d.−CH=CH2, −CH3, −C≡CH, −Harrow_forward3. Classify the alcohols shown below as primary, secondary, or tertiary. A) secondary B) primary C) tertiary 1) CH3 - CH2 - OH 2) CH3 | CH3 - C - CH2 - OH | CH3 3) OH | CH3 - CH - CH3 4) 5) CH3 | CH3 - C - CH2 - CH3 | OH 4. Identify the product, if any, that would form in each of the following reactions. A) CH3 - CH3 B) CH3 - CH2 - CH3 C) OH | CH3 - C H - CH3 D) O CH3 - C - OH E) CH3 - CH2 - OH 1) O CH3 – C - H 2) O CH3 - C - CH3 + H2 3) O CH3 - C - H + H2arrow_forward
- the context is "Cosmone is a molecule used by fragrance manufacturers to provide a rich and elegant musky essence to many perfumes. Cosmone has the molecular formula C15H26O." I need help on part (e) and (f).arrow_forwardRank the following compounds according to increasing positive character of the carbon atom. least positive C Most positive C CH3F CH3OH CH3Li CH3I CH3CH3 CH3NH2arrow_forwardDetermine the compound (name or structure) from the data. Explain features from each data Molecular formula: C3H8Oarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
