
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.47E
The following compounds are cyclic acetals or ketals. Write structural formulas
for the hydrolysis products.
a.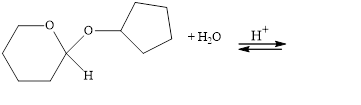
b.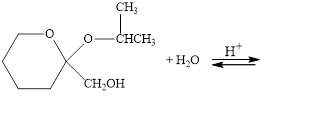
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
draw the organic product of the given reaction
name the organic product
What reagents are used in the esterification of Alcohols and Phenols?
a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride
b. Write the reaction involved in Phenol and Acetyl Chloride
What is the IUPAC name of the structure?
a. ethanoic acid
b. butanoic acid
c. methyl propanoate
d. ethyl propanoate
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 14 - Prob. 14.1ECh. 14 - Prob. 14.2ECh. 14 - Identify each of the following compounds as an...Ch. 14 - Identify each of the following compounds as an...Ch. 14 - Prob. 14.5ECh. 14 - Prob. 14.6ECh. 14 - Prob. 14.7ECh. 14 - Prob. 14.8ECh. 14 - Draw structural formulas and give IUPAC names for...Ch. 14 - Draw structural formulas and give IUPAC names for...
Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Prob. 14.13ECh. 14 - Prob. 14.14ECh. 14 - Prob. 14.15ECh. 14 - Explain why propane boils at 42C, whereas ethanal,...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Prob. 14.19ECh. 14 - Prob. 14.20ECh. 14 - Prob. 14.21ECh. 14 - Prob. 14.22ECh. 14 - Prob. 14.23ECh. 14 - Prob. 14.24ECh. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following structures as a cyclic...Ch. 14 - Label each of the following structures as a...Ch. 14 - What two functional groups react to form the...Ch. 14 - Hemiacetals are sometimes referred to as potential...Ch. 14 - Complete the following statements: a. Oxidation of...Ch. 14 - Prob. 14.32ECh. 14 - Prob. 14.33ECh. 14 - Prob. 14.34ECh. 14 - Prob. 14.35ECh. 14 - Not all aldehyde give a positve Bendicts test....Ch. 14 - A stockroom assistant prepares three bottles, each...Ch. 14 - Glucose, the sugar present within the blood, gives...Ch. 14 - Fructose, present with glucose in honey, reacts...Ch. 14 - Prob. 14.40ECh. 14 - Prob. 14.41ECh. 14 - Complete the following equations. If no reaction...Ch. 14 - Complete the following equations. If no reaction...Ch. 14 - Describe the products that result when hydrogen...Ch. 14 - Prob. 14.45ECh. 14 - Draw structural formulas for the products of the...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - Write equations to show how the following...Ch. 14 - Prob. 14.50ECh. 14 - Identify the most important aldehyde and ketone...Ch. 14 - Using Table 14.3, name an aldehyde or ketone used...Ch. 14 - Prob. 14.53ECh. 14 - CH3COH(O)CH3COOHacetaldehydeaceticacid You need to...Ch. 14 - The addition of water to aldehydes and ketones...Ch. 14 - Prob. 14.56ECh. 14 - Formaldehyde levels above 0.10mg/1000L of ambient...Ch. 14 - In the IUPAC name for the following ketone, it is...Ch. 14 - Why can formaldehyde (CH2O) be prepared in the...Ch. 14 - Other addition reactions of aldehydes occur....Ch. 14 - Prob. 14.61ECh. 14 - Prob. 14.62ECh. 14 - Vanilla flavoring is either extracted from a...Ch. 14 - Prob. 14.64ECh. 14 - The use of acetone in laboratory experiments must...Ch. 14 - Prob. 14.66ECh. 14 - Prob. 14.67ECh. 14 - Which of the following would be classified as a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardGive the structure of an alcohol that could be used to prepare each of the following compounds: a. b. c.arrow_forwardWrite the chemical equations for tollen's test reaction for (Use structural formula) a. n-hexane b. cyclohexene c. benzene d. toluenearrow_forward
- This carboxylic salts are effective ingredient against yeast and molds in beverages, jams, pie fillings and ketchup a. benzoates b. sorbates c. acetates d. propionatesarrow_forwardWhat type of product should be formed if butanol went through an oxidation reaction to completion? (A) carboxylic acid (B) ketone (C) no product formed (D) aldehydearrow_forwardWhat is an acetal?arrow_forward
- Acid Alcohol Odor Structure salicylic acid methanol wintergreen ? anthranilic acid methanol Grape ?arrow_forwardWhat is Hydrolysis of ethyl acetate? And what are those example of itarrow_forwarda.) Look up the structure of salicylic acid and the structure of acetic anhydride. Indicate where the two molecules would attach to form aspirin (acetylsalicylic acid). See if you can predict the remaining product in the synthesis. b.) What carboxylic acid and what alcohol react to produce propyl acetate (pear flavoring/smell)?arrow_forward
- Indole and pyridine rings are found in alkaloids.a. Sketch each ring.b. Name one compound containing each of the ring structures and indicate its use.arrow_forwardA chemist wants to make an ester from conversion of alcohol or phenol, what can he used? a. benzamide b. acetic anhydride c. maleic anhydrate d. nonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY