
(a)
Interpretation:
The given compound needs to be labelled as water soluble or insoluble.
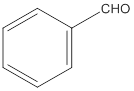
Concept Introduction:
The main rule which is used to determine the solubility of a compound in a given solution is that "like dissolves like". Therefore, the polar compounds will be soluble in polar solvents while the non-polar compounds will be soluble in non-polar solvents.
(b)
Interpretation:
The given compound needs to be labelled as water soluble or insoluble.
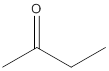
Concept Introduction:
The main rule which is used to determine the solubility of a compound in a given solution is that "like dissolves like". Therefore, the polar compounds will be soluble in polar solvents while the non-polar compounds will be soluble in non-polar solvents.
(b)
Interpretation:
The given compound needs to be labelled as water soluble or insoluble.

Concept Introduction:
The main rule which is used to determine the solubility of a compound in a given solution is that "like dissolves like". Therefore, the polar compounds will be soluble in polar solvents while the non-polar compounds will be soluble in non-polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- 45. Ethanol is a polar compound that readily dissolve in water, so it is A) immiscible SERVOTRONT OF Some ma 8arrow_forwardwhich organic compound dissolves in water? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanol which organic compound dissolves in 10% NaOH? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanolarrow_forwardConsider the structure of Vitamin A1 (C20H300). Vitamin A1 should be CH3 CH3 CH3 CH2OH H2C H2Č. C' H2 C. CH3 CH3 Periodic Table and Datasheet Select one: Oa. insoluble in water and in oil O b. less soluble in water than in oil O c. equally soluble in water and in oil O d. more soluble in water than in oil CH CH HC CH CH HCarrow_forward
- what is/are the major products) of this dehydration reaction? OH H-SO heat +. A. +. B. C. D.arrow_forwardWhat makes soluble diethyl ether and insoluble diethyl ether compounds soluble in waterarrow_forwardi. Why is the boiling point of the aldehyde greater than that of the alkane? ii. Why is the boiling point of alcohol the highest? iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forward
- 4. what makes acetone miscible with water?5. what makes ethyl acetate immiscible with water?arrow_forwardClassify the ff. alcohol 1°, 2°, 3°.arrow_forward1. Is Hexane and Benzene soluble in each other? 2. Is Hexane and 1-hexene soluble in each other? 3. Is Bromine (Br2) soluble in water?arrow_forward
- -Which substance has the greatest tendency to participate in a chemical reaction with small chain alcohol in an acidified chemical environment? a. Substance A b. Substance B c. Substance C d. Substance D e. Substance E -Which substance is expected to turn blue litmus paper into red color? a. Substance A b. Substance B c. Substance C d. Substance D e. Substance E -The physical properties of the substances are influenced by intermolecular forces of attraction. Which substance(s) are predominantly influenced by the intermolecular association of the molecules via hydrogen bonding? a.Substance A only b. Substance A and C c. Substance A, B, and C d.Substance A, B, C, and D -Aside from London dispersion forces, which substance do you expect dipole interactions to be effective? a. Substance A and B b. Substance B and D c. Substance A and C d. Substance B and E -Which substance will exhibit the lowest melting point? a. Substance A b. Substance B c. Substance C d. Substance D e. Substance E…arrow_forward78. The solvents, whether they have high or low melt ing and boiling points, or whether they react with other molecules. cetermine whether organic compounds are soluble in polar or non-polararrow_forwardwhat makes acetone,Ethanol,and Sucrose compounds soluble in water?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

