
a)
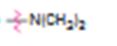
Interpretation:
Whether N,N-dimethylamino group is an activator or deactivator and whether it is a o-, p-director or m-director is to be stated.
Concept introduction:
In
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether N,N-dimethylamino group is an activator or deactivator and whether it is a, o-, p-director or m-director.
b)
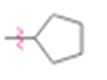
Interpretation:
Whether cyclopentyl group is an activator or deactivator and whether it is an o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether cyclopentyl group is an activator or deactivator and whether it is an o-, p-director or m-director.
c)
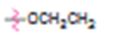
Interpretation:
Whether ethoxy group is an activator or deactivator and whether it is an o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether ethoxy group is an activator or deactivator and whether it is an o-, p-director or m-director.
d)
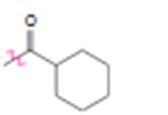
Interpretation:
Whether the carbonyl group is an activator or deactivator and whether it is a o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether carbonyl group is an activator or deactivator and whether it is a o-, p-director or m-director.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- The -Cl group is considered a .... Ortho/Para Directing Activator Ortho/Para Directing Deactivator Meta Directing Deactivatorarrow_forwardPropose a multistep synthesis for each of the intermediates / products in the following questions. Show all relevant reagents, solvents, and reaction conditionsarrow_forwardAzo coupling reaction. Provide a mechanism with intermediates and arrowsarrow_forward
- Discuss comprehensively why -Cl and -NO2 aredeactivators and why -Cl is a weak and -NO2 astrong deactivator?arrow_forwardgive a short explanation on what makes something activators or deactivators and WHY?arrow_forwardList the following in increasing order of nucleophilic strength. CH3COO-, H2O, CH3O-, OH-, CH3CH20-arrow_forward
- Identify which substitution mechanism best fits the following statement: The reaction proceeds through a concerted mechanism. A) SN1 B) SN2arrow_forwardPredict the major product for the following reaction and in the box provided, write thecorresponding compound letter (A, B, C, or D) or write NR if no reaction is expected to occur.arrow_forwardIdentify the leaving group for a potential elimination of the following compounds. Compare the leaving group activities for A and K. Which of the following compounds cannot be subjected to elimination? Explain.arrow_forward
- Identify the mechanistic pathways respectively for the products in the following reactionarrow_forwardThe -NHCOCH3 group is considered a .... Ortho/Para Directing Activator Ortho/Para Directing Deactivator Meta Directing Deactivatorarrow_forwardPropose multistep syntheses for each of the following. Explain the synthetic purpose of each step in your pathway.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

