
a)
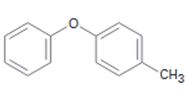
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
b)
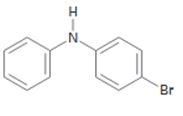
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions both -NH- and halogens are ortho and para directing as they stabilize the carbocation intermediates for these attacks. If two alternate options exist, the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
c)
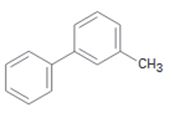
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, both aryl and alkyl are ortho and para directing as they stabilize the carbocation intermediates for these attacks. If two alternate options exist, the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
d)
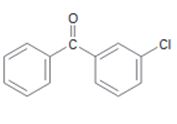
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions –C=O group is meta directing while halogens are ortho and para directing. If two alternate options exist the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- List the following order of increasing reactivity in an SN1 reactionarrow_forwardWhich of the following two compounds do you expect to be a better electrophile, and why?arrow_forwardHighlight each of the positions on the benzene ring that you expect will be most likely to react by electrophilic aromatic substitution. Am I correct?arrow_forward
- Give the correct order of the given compounds in order of decreasing reactivity towards electrophilic aromatic substitution.arrow_forwardWhich of A to E correctly shows the structure of the major organic product obtained from the reaction of the epoxide shown below?arrow_forwardWhat would you expect to be the major product C of the following sequence of reactions?arrow_forward
- What starting material is required in order to synthesize each of the following compounds by ring-closing metathesis?arrow_forwardThe reaction below exhibits electrophilic aromatic substitution: TRUE OR FALSEarrow_forwardWhich compound shown below would undergo nucleophilic substitution via addition/elimination the most quickly?arrow_forward
- Which of A to E correctly shows the structure of the major organic product obtained from the ozonolysis reaction shown below?arrow_forwardGive the structures of compounds K through P in the following series of reactions.arrow_forwardOrder the following compounds in decreasing order of reactivity in an electrophilic aromatic substitution reaction:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





