
a)
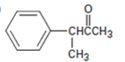
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared is to be given.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of aTo give:
Using an alkylation reaction as the key step how to prepare the compound shown.
b)
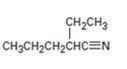
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of a ketone, ester or nitrile by a SN2 reaction of the enolate ion with an alkyl halide. Thus by looking at α- carbon of the product the alkyl halide to be used in the reaction can be identified. The enolate ion can be produced by using lithium diisopropylamide (LDA).
To give:
Using an alkylation reaction as the key step how to prepare the compound shown.
c)
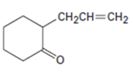
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of a ketone, ester or nitrile by aSN2reaction of the enolate ion with an alkyl halide. Thus by looking at α- carbon of the product the alkyl halide to be used in the reaction can be identified. The enolate ion can be produced by using lithium diisopropylamide (LDA).
To give:
Using an alkylation reaction as the key step how to prepare the compound shown.
d)
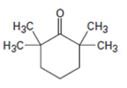
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of a ketone, ester or nitrile by a SN2 reaction of the enolate ion with an alkyl halide. Thus by looking at α- carbon of the product the alkyl halide to be used in the reaction can be identified. The enolate ion can be produced by using lithium diisopropylamide (LDA).
To give:
Using an alkylation reaction as the key step how to prepare the compound shown.
e)
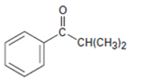
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of a ketone, ester or nitrile by a SN2 reaction of the enolate ion with an alkyl halide. Thus by looking at α- carbon of the product the alkyl halide to be used in the reaction can be identified. The enolate ion can be produced by using lithium diisopropylamide (LDA).
To give:
Using an alkylation reaction as the key step how to prepare the compound shown.
f)
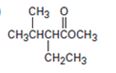
Interpretation:
Using an alkylation reaction as the key step how the compound shown can be prepared.
Concept introduction:
An alkylation reaction is used to introduce a methyl or a primary alkyl group in α- position of a ketone, ester or nitrile by a SN2 reaction of the enolate ion with an alkyl halide. Thus by looking at α- carbon of the product the alkyl halide to be used in the reaction can be identified. The enolate ion can be produced by using lithium diisopropylamide (LDA).
To give:
Using an alkylation reaction as the key step how to prepare the compound shown.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Show how you would accomplish the following syntheses. ) hexan@1@ol S 2-hydroxyheptanoic acidarrow_forwardShow how you would use direct alkylation to synthesize the following compounds.(a) benzyltrimethylammonium iodidearrow_forwardShow how you would synthesize the following esters from appropriate acyl chloridesand alcohols. (c) benzyl benzoate (d) cyclopropyl cyclohexanecarboxylatearrow_forward
- 4. propose a mechanism for the following reaction (don't go over 18 electrons!). be sure to use curved arrows, state the electron count of each intermediate, and name the reaction at each step.arrow_forwardShow how you would accomplish the following syntheses.benzene ¡ p@methoxybenzaldehydearrow_forwardPropose a mechanism to show how 3,3-dimethylbut-1-ene reacts with dilute aqueousH2SO4 to give 2,3-dimethylbutan-2-ol and a small amount of 2,3-dimethylbut-2-ene.arrow_forward
- Propose a multi-step synthesis of the target molecule shown at the right, using the starting materials on the left and any other reagents you need. Show the reagents needed for each step and the product of each step. You will need 4 reaction arrows.arrow_forwardHow would you synthesize the methyl ketone shown below via the acetoacetic ester synthesis? You must show the reactions/reagents used in their correct orderarrow_forwardIf methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you would carry out the following transformation, and propose a mechanism for the ester-forming step.arrow_forward
- Show how Wittig reactions might be used to synthesize the following compounds. Ineach case, start with an alkyl halide and a ketone or an aldehyde.(a) Ph¬CH“C(CH3 )2 (b) Ph¬C(CH3 )“CH2(c) Ph¬CH“CH¬CH“CH¬Pharrow_forwardHow would you carry out the following synthesis of cyclohexane cyclohexanol cyclohexanearrow_forwardpredict the products for the following reaction and propose an electron pushing mechanismarrow_forward
