
Concept explainers
a)
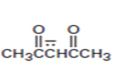
Interpretation:
The resonance structures for the anion shown are to be drawn.
Concept introduction:
Resonance forms differ only in the position of the π bonding and nonbonding pair of electrons. The position and hybridization of the atoms do not change from one resonance form to the other. Octet rule is applied while writing the different resonance forms.
b)
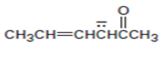
Interpretation:
The resonance structures for the anion shown are to be drawn.
Concept introduction:
Resonance forms differ only in the position of the π bonding and nonbonding pair of electrons. The position and hybridization of the atoms do not change from one resonance form to the other. Octet rule is applied while writing the different resonance forms.
c)
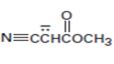
Interpretation:
The resonance structures for the anion shown are to be drawn.
Concept introduction:
Resonance forms differ only in the position of the π bonding and nonbonding pair of electrons. The position and hybridization of the atoms do not change from one resonance form to the other. Octet rule is applied while writing the different resonance forms.
d)
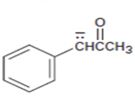
Interpretation:
The resonance structures for the anion shown are to be drawn.
Concept introduction:
Resonance forms differ only in the position of the π bonding and nonbonding pair of electrons. The position and hybridization of the atoms do not change from one resonance form to the other. Octet rule is applied while writing the different resonance forms.
e)
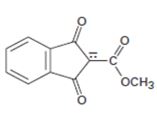
Interpretation:
The resonance structures for the anion shown are to be drawn.
Concept introduction:
Resonance forms differ only in the position of the π bonding and nonbonding pair of electrons. The position and hybridization of the atoms do not change from one resonance form to the other. Octet rule is applied while writing the different resonance forms.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Organic Chemistry
- Electrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the a position. Predict themajor products of the reactions of naphthalene with the following reagents. cyclohexanol and BF3arrow_forwardWhich Nitrogen in the following compound is more nucleophilic? Explain why using electron density. Show 2 resonance structures for the compound.arrow_forwardCompound X (molecular formula C10H12O) was treated with NH2NH2,−OH to yield compound Y (molecular formula C10H14). Based on the 1H NMR spectra of X and Y given below, what are the structures of X and Y?arrow_forward
- Propose a structure for each of the following two isomers with formula C6H14 given their 1H-NMR spectra. Isomer A: δ = 0.84 (d, 12 H), 1.39 (septet, 2H) ppm Isomer B: δ = 0.84 (t, 3 H), 0.86 (t, 9H), 1.22 (q, 2H) ppmarrow_forwardElectrophilic aromatic substitution usually occurs at the 1-position of naphthalene, also called the a position. Predict themajor products of the reactions of naphthalene with the following reagents.(a) HNO3, H2SO4arrow_forward1, 6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at -0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.arrow_forward
- Following is the structural formula of Surfynol, a defoaming surfactant. Describe the synthesis of this compound from acetylene and a ketone. How many stereoisomers are possible for Surfynol?arrow_forwardPropose a structure for the compound, C4H8O, that has the following 1H NMR spectrumarrow_forwardAddition of mesitylene to the strongly acidic solvent HF / SbF5 at -45 °C gives a new species which shows a 1H-NMR resonance at δ 4.67 (2H). Draw the structure of this species.arrow_forward
- How can you prepare the following compounds with benzene as one of the starting materials?arrow_forwardThe compound with the 1H NMR spectrum shown is known to be highly reactive toward electrophilic aromatic substitution. Identify the compound.arrow_forwardHow can you prepare the following compound with benzene as one of the starting materials?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

