
a)
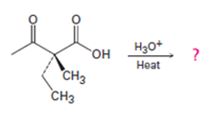
Interpretation:
Whether the
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
b)
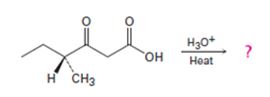
Interpretation:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not is to be stated. A mechanism to explain the formation of the ketone is to be proposed.
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Organic Chemistry
- The compounds commonly known as “amino acids” are actually a-aminocarboxylic acids . What carbonyl compounds should be used to synthesize the two amino acids shown here?arrow_forwardLidocaine synthesis consists of two steps, 2,6-dimethylaniline (1) is treated with chloroacetyl chloride under weakly basic conditions to selectively generate amide 2. The second step of the synthetic sequence involves the alkylation of diethylamine by the alkyl chloride group in 2. Based on this can you please answer the questions below! 1. Identify all electrophilic sites in chloroacetyl chloride. 2. Assuming that the yield of your amidation reaction was 100%, how many mole equivalents of diethylamine are you using in the second step of this synthesis? 3. Indicate two differences between the IR spectra of lidocaine (3) and 2,6-dimethylaniline (1). 4. Explain how you will be able to tell that the second step of the synthetic route was successful.arrow_forwardA ketone undergoes acid-catalyzed bromination, acid-catalyzed chlorination, racemization, and acid-catalyzed deuterium exchange at the alpha-carbon. All of these reactions have similar rate constants. What does this tell you about the mechanisms of these reactions?arrow_forward
- An elimination step is involved in the synthesis of aromatic amino acids. We can also envision this occurring in a laboratory setting with a strong base. Provide a 1 step mechanism for this decarboxylation reaction. Note: under strongly basic conditions, hydroxide is a reasonable leavinggroup.arrow_forwardOne of the later steps in glucose biosynthesis is the isomerization of fructose 6-phosphate to glucose 6-phosphate. Propose a mechanism, using acid or base catalysis as needed.arrow_forwardWhich of the following substituent is an ortho/para-directing deactivator? a amino group b halogen group c phenyl group d carboxyl grouparrow_forward
- Suggest the synthesis pathways (not more than two reactions) for the formation of any ketone compound by using Compound N as precursor. You can use any reactions that is relatable.arrow_forwardPropose a mechanism for the following transformation which occurs in this reaction? (provided in the image)arrow_forwardHello, using the malonic ester synthesis, write the synthesis of the following compound by showing its mechanism.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

