
Certain solid substances, known as hydrated compounds, have well-defined molecular ratios of water to some other species. For example, calcium sulfate dihydrate (commonly known as gypsum, CaSO42H2O), has 2 moles of water per mole of calcium sulfate; alternatively, it may be said that 1 mole of gypsum consists of 1 mole of calcium sulfate and 2 moles of water. The water in such substances is called water of hydration. (More information about hydrated salts is given in Chapter 6.)
In order to eliminate the discharge of sulfuric acid into the environment, a process has been developed in which the acid is reacted with aragonite (CaCO3) to produce calcium sulfate. The calcium sulfate then comes out of solution in a crystallizer to form a slurry (a suspension of solid particles in a liquid) of solid gypsum particles suspended in an aqueous CaSO4 solution. The slurry flows front tlte crystallizer to a filter in which the particles arc collected as a filter cake. The filter cake, which is 95.0 wt% solid gypsum and the remainder CaSO4 solution, is fed to a dryer in which all water (including the water of hydration in the crystals) is driven off to yield anhydrous (water-free) CaSO4 as product A flowchart and relevant process data arc given below.
Solids content of slurry leaving crystallizer 0.35 kg CaSO4-2H2O/L slurry CaSO4 content of slurry liquid: 0.209g CaSO4/100g H2O Specific gravities: CaSO4-2H2O(s), 2.32; liquid solutions, 1.05
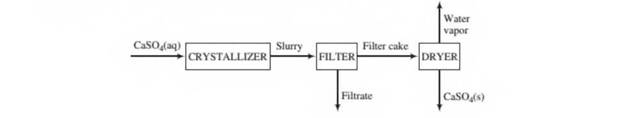
- Briefly explain in your own words the functions of the three units (crystallizer, filter, and dryer).
- Take a basis of one liter of solution leaving the crystallizer and calculate tlte mass (kg) and volume (L) of solid gypsum, the mass of CaSO4 in the gypsum, and the mass of CaSO4 in the liquid solution.
- Calculate the percentage recovery of CaSO4—that is, the percentage of the total CaSO4(precipitated plus dissolved) leaving the crystallizer recovered as solid anhydrous CaSO4.
- List five potential negative consequences of discharging H2SO4 into the river passing the plant.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Starting Out with C++ from Control Structures to Objects (8th Edition)
Structural Analysis (10th Edition)
Experiencing MIS
C How to Program (8th Edition)
- The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forwardConsider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forwardCarbon dioxide emissions associated with a one-night stay in a hotel room are calculated at 28.98 kg of CO2 per room day for an average hotel. The 250 rooms of your hotel are all occupied for two days during a college football game. How much CO2 did the guests and hotel release into the atmosphere? Round your answer to the nearest whole number. kgsarrow_forward
- Hexamethylenediamine (CH16N2) is one of the starting materials for the production of nylon. It can be prepared from adipic acid (CH1004) by the following overall reaction: C6H1004(1) + 2 NH;(g) + 4 H2(g) – CH16N2(1) + 4 H,0()arrow_forward2. An experiment to determine the carbon-sequestration capacity of a poui tree determined that in 1 year, the mature tree could remove 11,455 L (measured at room temperature and pressure) of carbon dioxide from the air, using the process in Q.1(d) to produce glucose. What mass of glucose would the poui tree have produced doing this?arrow_forwardAspirin, C6H4 (CO2H) (CO2CH3), can be prepared in the chemistry laboratory by the reaction of salicylic acid, C6H4 (CC2H) (OH), with acetic anhydride, (CH3CO)2O 2C6H4(CO2H)(OH)+(CH3CO)2O>>C6H4(CO2H)(CO2CH3)+H2O What volume of acetic anhydride (density, 1.0820 g/cm3) is required to produce 1.00 kg of aspirin, assuming a 100% yield?arrow_forward
- Ammonia (NH3) is an important compound since it is used in large amounts for the manufacture of nitrogenous fertilizers, nylon and many other important compounds. It is manufactured industrially by the catalytic reaction between nitrogen between nitrogen and hydrogen, according to the following equation, N2(g) + H2(g) = NH;(g) When 1.20 mol nitrogen and 1.20 mol hydrogen are mixed together in a closed vessel, 30% of the nitrogen is converted into ammonia. a Calculate the moles of nitrogen gas that will be present in the vessel? (only 3 decimal places) mol b. Calculate the moles of hydrogen gas that will be present in the vessel? (only 3 decimal places) mol c. Calculate the moles of ammonia gas that will be present in the vessel? (only 3 decimal places) mol d What is the number of moles of nitrogen gas that has reacted? (only 3 decimal places) mol e. What mass of ammonia can be produced from the limiting reactant? (only 3 deceimal places)arrow_forwardAutomobiles are often implicated as contributors to global warming because they are a source of the greenhouse gas CO₂. How many pounds of CO₂ would your car release in a year if it was driven 110. miles per week? Gasoline is a complex mixture of hydrocarbons. In your calculations, assume that gasoline is isooctane (molecular formula C8H18) and that it is burned completely to CO2 and H₂O in the engine of your car. Also assume that the car averages 24.0. miles per gallon and that the density of isooctane is 0.692 g/cm³. Pounds of CO2 released = lbsarrow_forward4. A common experiment at Science Fairs is the demonstration of a volcano using a mixture of baking soda, NaHCO3(s), and vinegar, CH₂COOH(aq), with the reaction: NaHCO3(s) + CH3COOH(aq)- CO2(g) + H₂O(l) + Nat(aq) + CH3COO(aq) where the heavier CO2 gas displaces the liquid mixture to the top of the container to simulate lava overflowing. If the volume of the reaction vessel is 0.500 L and the pressure is 0.997 atm and 298 K. given 50.5 ml of 0.351 M CH3COOH() in water, what mass of NaHCO3(s) is required to fill the bottle with CO2 gas?arrow_forward
- Carbon dioxide gas, CO2(g), is generated in the combustion of a sample of ethane (C2H6). This CO2 is all bubbled into aqueous barium hydroxide to be absorbed to produce 0.506 g of solid barium carbonate precipitate. How many grams of ethane (C2H6) were initially burned? The absorption-precipitation reaction is given by [CO2(g) + Ba(OH)2(aq) BaCO3(s) + H2O(l)].arrow_forwardThe fermentation of sugar to produce ethyl alcohol occurs by the following reaction: C6H12O6 (s) yeast 2C₂H5OH() + 2CO₂ (g) What mass of ethyl alcohol can be made from 1.57 kg of sugar?arrow_forwardHydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: 2NH3(g) + 3O2(g) + 2CH4(g) →PI 2HCN(g) + 6H2O(g) Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 11.5 g NH3, 12.0 g O2, and 10.5g CH4, what is the ma_ximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



