
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.30P
The structure of 1,1,2,2-tetraiododisilane is shown here. (Reference: T. H. Johansen. K.Hassler. G. Tekautz, K. Hagen, J. Mol. Struct., 2001, 598, 171.)
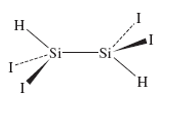
a What is the point group of this molecule?
b. Predict the number of IR-active
c. Predict the number of Raman-active
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
One of the first compound synthesized where Xenon is a part of a cationic complex is XeF3+ SbF6-. To what point group does the cationic and anionic complex belong, respectively?
A. C2v, Oh
B. C3v, Oh
C. D3h, Oh
D. none of the above is the orrect point group assignment
E. D3h, C5v
Given molecule: PCl3 and NH3
1. Identify point group
2. Draw Lewis structure, and identify molecular shape according to VSEPR theory
3. Indicate coordinates of each atom
4. Use projection operator method to construct MO diagram
5. Sketch all atomic and group orbitals (show the phase/polarity (as necessary))
6. Indicate the symmetry of each atomic and group orbitals
7. Sketch the molecular orbitals that satisfy all the conditions for overlap (with corresponding anti-bonding MOs), and all the non-bonding MOs.
8. Sketch the final MO diagram.
The molecule with simple formula N2F2 has several isomers; two of these isomers have central nitrogens, with a terminal fluorine on each nitrogen. Show the Lewis Dot Diagram.
For the cis isomer :
What is the point group? (include formal charges, if any). Write also the main symmetry elements
How many IR bands bands would you expect if vib = 3A1 + A2 +2B2?
For the trans isomer :
What is the point group? (include formal charges, if any). Write also the main symmetry elements
How many IR bands bands would you expect if vib = 3Ag + Au +2Bu?
Chapter 4 Solutions
Inorganic Chemistry
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Find all the symmetry elements in the following...Ch. 4.2 - Use the procedure described previously to verify...Ch. 4.3 - Prob. 4.4ECh. 4.3 - Verify the transformation matrices for the E and...Ch. 4.3 - Prepare a representation flowchart according to...Ch. 4.4 - Which point groups are possible for chiral...Ch. 4.4 - Write the corresponding 99 transformation matrices...Ch. 4.4 - Using the x, y, and z coordinates for each atom in...Ch. 4.4 - Reduce the following representations to their...
Ch. 4.4 - Prob. 4.11ECh. 4.4 - Analysis of the x, y, and z coordinates of each...Ch. 4.4 - Determine the number of IR-active CO stretching...Ch. 4.4 - Prob. 4.14ECh. 4 - Determine the point groups for a. Ethane...Ch. 4 - Determine the point groups for a. Ethylene b....Ch. 4 - Determine the point groups for a. Acetylene b....Ch. 4 - Determine the point groups for a. Naphthalene b....Ch. 4 - Determine the point groups for a. 1,1’ ...Ch. 4 - Determine the point groups for a. Cyclohexane...Ch. 4 - Determine the point groups for a. A sheet of...Ch. 4 - Determine the point groups for a. A flat oval...Ch. 4 - Determine the point groups for a. A triangular...Ch. 4 - Determine the point groups for the examples of...Ch. 4 - Determine the point groups of the molecules in the...Ch. 4 - Determine the point groups of the molecules and...Ch. 4 - Determine the point groups of the following atomic...Ch. 4 - a. Show that a cube has the same symmetry elements...Ch. 4 - Suppose an octahedron can have either yellow or...Ch. 4 - What point groups are represented by the symbols...Ch. 4 - Prob. 4.17PCh. 4 - Determine the point groups for the following flags...Ch. 4 - Prepare a representation flowchart according to...Ch. 4 - For trans-1,2-dichloroethylene, which has C2h...Ch. 4 - Ethylene has D2h symmetry. a. List all the...Ch. 4 - Using the D2d character table, a. Determine the...Ch. 4 - Reduce the following representations to...Ch. 4 - For D4h symmetry use sketches to show that dxy...Ch. 4 - Prob. 4.25PCh. 4 - XeOF4 has one of the more interesting structures...Ch. 4 - Repeat the procedure from the previous problem,...Ch. 4 - For the following molecules, determine the number...Ch. 4 - Prob. 4.29PCh. 4 - The structure of 1,1,2,2-tetraiododisilane is...Ch. 4 - Both cis and trans isomers of IO2F4 have been...Ch. 4 - White elemental phosphorus consists of tetrahedral...Ch. 4 - Complexes of the general formula Fe(CO)5x( PR3)x...Ch. 4 - Prob. 4.35PCh. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Determine the point groups of the following...Ch. 4 - Prob. 4.40PCh. 4 - Determine the point groups of the following: a....Ch. 4 - Use the Internet to search for molecules with the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Structural isomers can have very different point groups. Determine the point groups of 1,4cyclohexadiene and 1,3cyclohexadiene, which both have the molecular formula C6H8.arrow_forwardDetermine the point groups of the following molecules. a Fe(CO)5, which has a trigonal bipyramidal structure, b CO32, which has three resonance structures that contribute to its overall shape, c The perfectly staggered conformation of ethane, d The perfectly eclipsed conformer of ethane.arrow_forward(a) Which is the acid and which is the base in the reaction? Explain using the appropriate acid / base definitions. (b) A proton is defined as a hard acid. What does this tell you about the properties of [Si9]4-? Explain. (c) [Si9]4- belongs to point group C4v. 1. Describe what will happen to the symmetry elements if one of the atoms is replaced with carbon, as seen in the following figures: (specify which symmetry elements are lost) 2. Is either A or B chiral? Explain. (d) Using Wade's rules, predict the cluster structure of [Si9]4-. Which borohydride cluster is identiical to the [Si9]4-cluster structure? explain.arrow_forward
- Please help Orbital and SALC symmetry Methane CH4 --- Point group = Td E, 8C3, 3C2, 6S4, 6σd I. What are the symmetries of the relevant orbital on C? II. What are the symmetries of the SALCs generated from the hydrogen orbitals? III. Use parts I and II to draw an MO diagram for CH4 IV. Use your MO diagram to sketch your prediction of the photoelectron spectrum for CH4arrow_forwardGive the point groups of the following molecules:arrow_forward( topic inorganic mossbauer spectroscopy ) plz answer in detail by drwaing electronic configurationnarrow_forward
- pavia Spectroscopy fifth edition page 278 ques 10 and page 281 ques 19arrow_forwardDiatomic molecules. The order of stability of molecular orbitals a) It is always the same for all b) It is almost the same as that of the starting atomic orbitals c) It follows from the appropriate correlation diagrams d) It is known exactly quantitatively.arrow_forwardDetermine the point groupof the following molecules Methane, Carbon tetra fluoride Boron tri fluoridearrow_forward
- Which of the following would be expected to have a dipole moment of zero on the basis of symmetry? Explain. a. SOCl2; b. SiF4; c. OF2.arrow_forwardWhat point groups result from the combination of two mirror planes oriented at 90° with respect to one another? Repeat for 60°, 45°, and 30°.arrow_forwardschematically show the pi electronic enery levels of hexatrienearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY