
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.4, Problem 4.12E
Analysis of the x, y, and z coordinates of each atom in
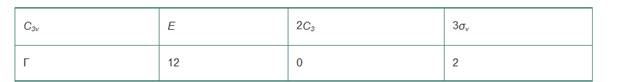
a. Reduce
b. Classify the irreducible representations into translational, rotational, and vibrational modes.
c. Show that the total number of degrees of freedom
d. Which vibrational modes are infrared active?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider a trigonal bipyramidal molecule XY5.
(i) To which point group does this molecule belong?
(ii) How many normal modes and how many stretching vibrations will this molecule exhibit?
(iii) Determine the irreducible representations of stretching vibrations of this molecule. Clearly show all your work.
(iv) Which of these vibrations stretching are IR and which are Raman active? Justify your choice
How many normal modes and how many stretching vibrations will this molecule exhibit
The structure of [NO3]-is either trigonal planar or trigonal pyramidal draw both possible structures and determine the point group for each.
Which irreducible representations represent the translational and rotational modes for each possible structure of [NO3]-?
Which irreducible representations correspond to the vibrational modes for each possible structure of [NO3]-? How many degrees of freedom would you expect for each structure of [NO3]-?
Calculate T-vibrationaland determine which modes you expect to be IR active for each structure.
Determine the number (and relative intensity) of IR-active C–O stretching vibrations for fac-[Mo(CO)3(NCMe)3]:
Proceed as follows...
Determine the point group of the molecule. Do not descend in symmetry.
Construct a reducible representation for the CO vibrations (ΓCO) -- [please explain how to determine the number of unshifted atoms]
Decompose ΓCO into its irreducible representation ΓCO,i.r.
Determine which are IR active (number of IR active modes)
Double degenerate modes (i.r. with E symmetry) are ≈ twice as intense as other modes, and
triply degenerate modes (i.r. with T symmetry) are ≈ three times as intense.
Chapter 4 Solutions
Inorganic Chemistry
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Find all the symmetry elements in the following...Ch. 4.2 - Use the procedure described previously to verify...Ch. 4.3 - Prob. 4.4ECh. 4.3 - Verify the transformation matrices for the E and...Ch. 4.3 - Prepare a representation flowchart according to...Ch. 4.4 - Which point groups are possible for chiral...Ch. 4.4 - Write the corresponding 99 transformation matrices...Ch. 4.4 - Using the x, y, and z coordinates for each atom in...Ch. 4.4 - Reduce the following representations to their...
Ch. 4.4 - Prob. 4.11ECh. 4.4 - Analysis of the x, y, and z coordinates of each...Ch. 4.4 - Determine the number of IR-active CO stretching...Ch. 4.4 - Prob. 4.14ECh. 4 - Determine the point groups for a. Ethane...Ch. 4 - Determine the point groups for a. Ethylene b....Ch. 4 - Determine the point groups for a. Acetylene b....Ch. 4 - Determine the point groups for a. Naphthalene b....Ch. 4 - Determine the point groups for a. 1,1’ ...Ch. 4 - Determine the point groups for a. Cyclohexane...Ch. 4 - Determine the point groups for a. A sheet of...Ch. 4 - Determine the point groups for a. A flat oval...Ch. 4 - Determine the point groups for a. A triangular...Ch. 4 - Determine the point groups for the examples of...Ch. 4 - Determine the point groups of the molecules in the...Ch. 4 - Determine the point groups of the molecules and...Ch. 4 - Determine the point groups of the following atomic...Ch. 4 - a. Show that a cube has the same symmetry elements...Ch. 4 - Suppose an octahedron can have either yellow or...Ch. 4 - What point groups are represented by the symbols...Ch. 4 - Prob. 4.17PCh. 4 - Determine the point groups for the following flags...Ch. 4 - Prepare a representation flowchart according to...Ch. 4 - For trans-1,2-dichloroethylene, which has C2h...Ch. 4 - Ethylene has D2h symmetry. a. List all the...Ch. 4 - Using the D2d character table, a. Determine the...Ch. 4 - Reduce the following representations to...Ch. 4 - For D4h symmetry use sketches to show that dxy...Ch. 4 - Prob. 4.25PCh. 4 - XeOF4 has one of the more interesting structures...Ch. 4 - Repeat the procedure from the previous problem,...Ch. 4 - For the following molecules, determine the number...Ch. 4 - Prob. 4.29PCh. 4 - The structure of 1,1,2,2-tetraiododisilane is...Ch. 4 - Both cis and trans isomers of IO2F4 have been...Ch. 4 - White elemental phosphorus consists of tetrahedral...Ch. 4 - Complexes of the general formula Fe(CO)5x( PR3)x...Ch. 4 - Prob. 4.35PCh. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Determine the point groups of the following...Ch. 4 - Prob. 4.40PCh. 4 - Determine the point groups of the following: a....Ch. 4 - Use the Internet to search for molecules with the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -What values of J are consistent with the terms 2P and 3D? How many states with different values of MJ correspond to each? -Describe the symmetry-adapted core and valence orbitals of H2O and H2S in the C2v point grouparrow_forwardPCl3 belong to C3V point group ? (b) Write down the totally symmetric representation of the point group of PCl3. (c) What is a symmetry-adapted linear combination of atomic orbitals? (d) Construct a linear-combination of 3s(Cl) atomic orbitals, ?1, such that ?1 belongs to the totally symmetric representation of the point group of PCl3. (e) Construct a linear-combination of 3p(Cl) atomic orbitals, ?2, such that ?2 belongs to the totally symmetric representation of the point group of PCl3.arrow_forwardHow many vibrational modes does an SO3 molecule have a) in the plane of the nuclei, b) perpendicular to the molecular plane? c) Classify them by their symmetry type.arrow_forward
- Which one isn’t not rotationally raman active? HCN, HF, Br2, CCL4arrow_forward1.Consider an XeOCl4 molecule. a) Determine the point group of the molecule. b) Determine a hybridization of the Xe atom. c) Determine a symmetry of the vibrational modes of the molecule. Please answer completelyarrow_forwardConsider the molecule CH3Cl. (a) To what point group does the molecule belong? (b) How many normal modes of vibration does the molecule have? (c) What are the symmetry species of the normal modes of vibration of this molecule? (d) Which of the vibrational modes of this molecule are infrared active? (e) Which of the vibrational modes of this molecule are Raman active?arrow_forward
- how can i get a no. of rotational modes of N2 no. of vibration modes of N2 no. of microwave active modes of N2 no. of infrared active modes of N2arrow_forwardWhat is the symmetry element corresponding to (a) Cn, (b) s, (c) i, (d) Sn? What is the symmetry operation corresponding to ?53?arrow_forwardHow do the rotation axes and planes of symmetry in cis- and trans-N2F2 differ? Why do CO2 and SO2 have different number of degrees of vibrational mode? Calculate the no of vibration modes of CO2. Which of these are IR or Raman Active?arrow_forward
- (I) Explain the law of mutual exclusion in IR and Raman spectroscopies using XeF4 as an exemplar molecule. (ii) Draw the orbitals that house the lone pairs on the O atom of a water molecule – and explain why each is a lone pair. (iii)Briefly explain how a symmetry adapted analysis of bonding in the water molecule yields the observed H-O-H bond angle.arrow_forwardGive the ground state electron configurations of(a) Li2 (b) Be2 (c) C2 .Use the symmetry-specific nomenclature appropriate for homonuclear diatomics. Example, Sg would stand for σg. Enclose each MO in parenthesis followed by the number of electrons in that MO, e.g., the entry (1Sg)2 would represent 1σg2. Letter P then is used to represent a pair of π MO's.arrow_forwardUsing its x, y, and z coordinates, construct the 15 x 15 matrix that describes the application of the S4 symmetry operation to square planar [PtCl 4]2- What is the character of this matrix?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
Gerade and Ungerade Molecular Orbitals. (SYMMETRY OF MOLECULAR ORBITALS); Author: Edmerls;https://www.youtube.com/watch?v=dPY-lT5LN60;License: Standard YouTube License, CC-BY
Symmetry and chemical bonding part – 5 Molecular orbital formation (CHE); Author: Vidya-mitra;https://www.youtube.com/watch?v=g-42GmpBu0I;License: Standard Youtube License