
(a)
Interpretation:
Structural formula of the organic product formed when butanal undergoes Tollen’s test has to be drawn.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
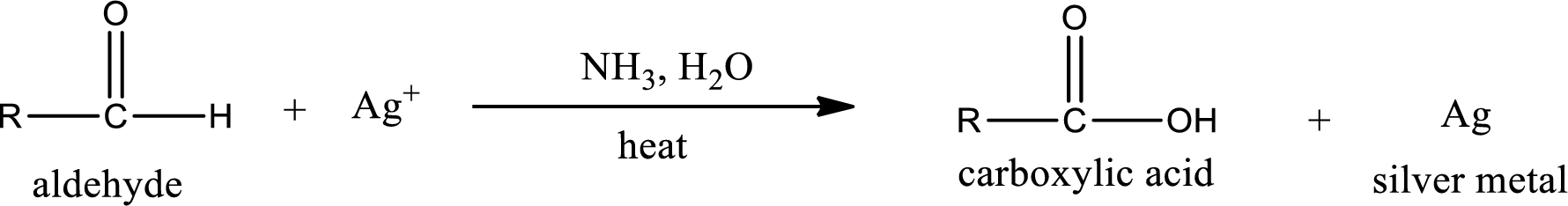
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
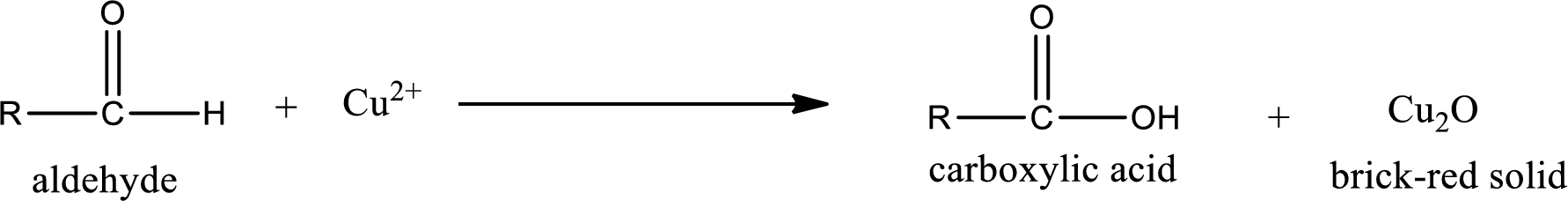
(a)
Answer to Problem 4.77EP
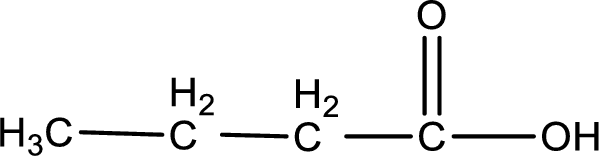
The structure of organic product obtained is,
Explanation of Solution
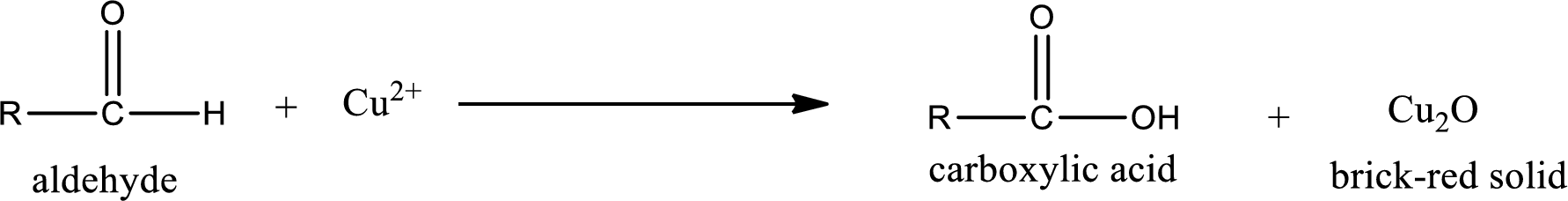
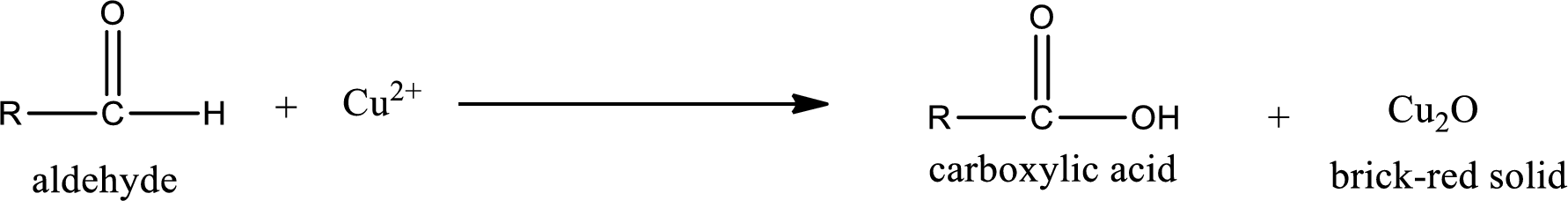
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
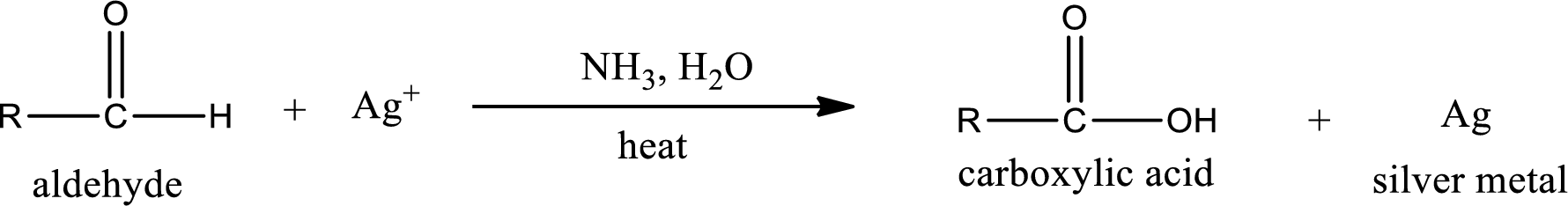
Given aldehyde is butanal and the structure can be given as shown below,
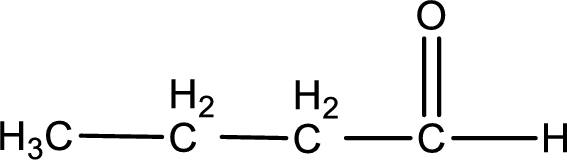
This on reaction with Tollen’s reagent gives carboxylic acid as the product. The structure of the organic product formed and the complete reaction can be given as shown below,
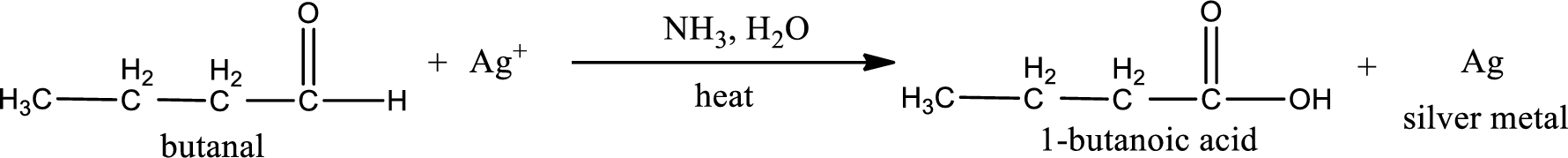
1-butanoic acid is formed from butanal when it undergoes Tollen’s test.
The structure of the organic product formed is drawn.
(b)
Interpretation:
Structural formula of the organic product formed when 2-butanone undergoes Tollen’s test has to be drawn.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
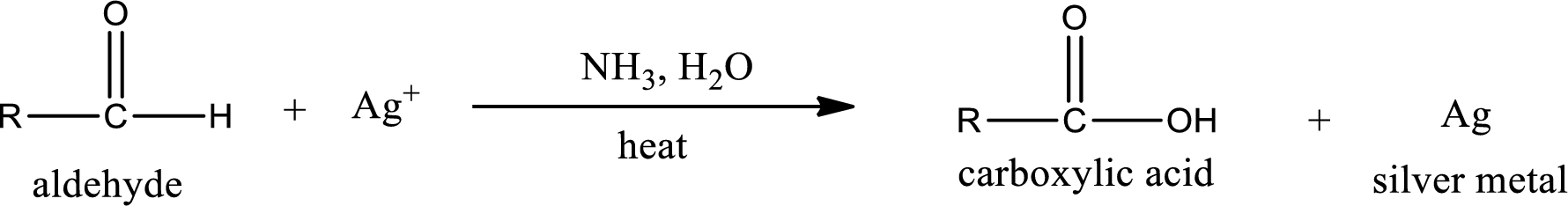
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
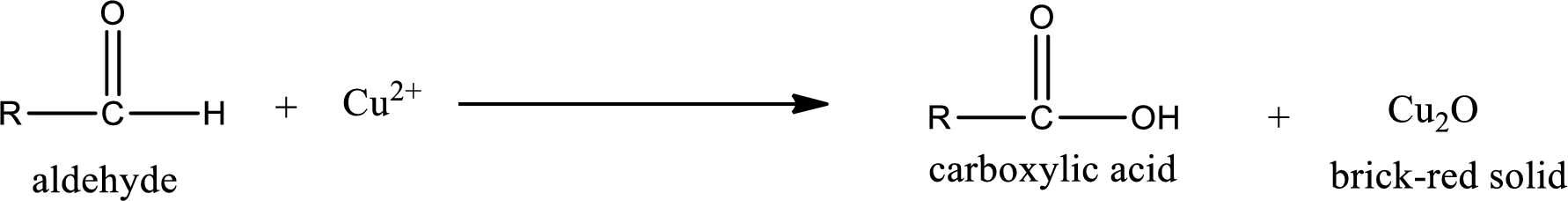
(b)
Answer to Problem 4.77EP
2-butanone does not undergo Tollen’s test.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
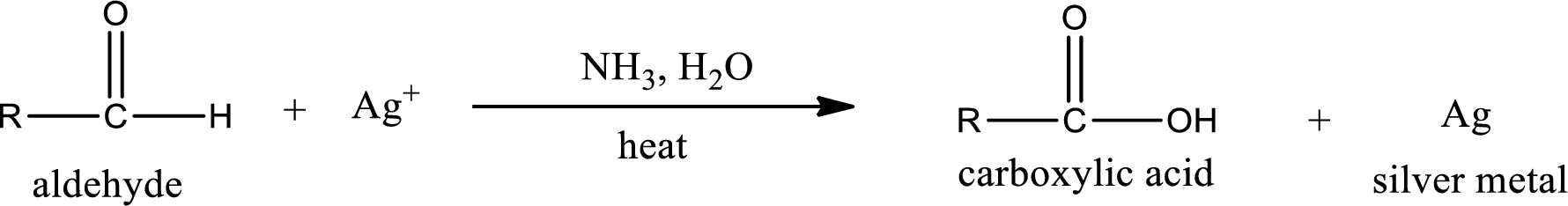
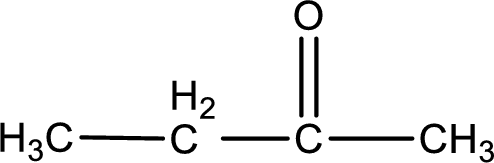
Given compound is a ketone that is 2-butanone and the structure can be given as shown below,
This on reaction with Tollen’s reagent does not give oxidized product. Therefore, no reaction takes place when 2-butanone reacts with Tollen’s reagent.
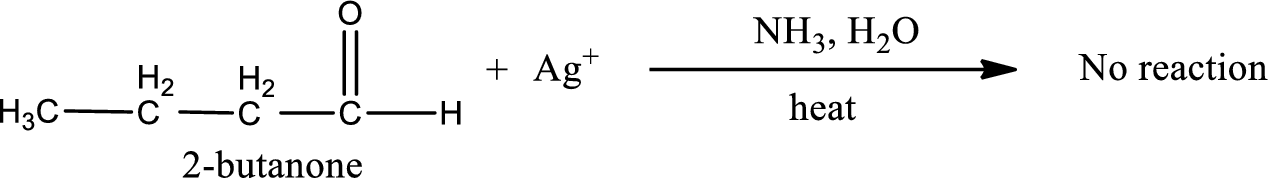
2-butanone does not react with Tollen’s reagent.
No reaction takes place when 2-butanone undergoes Tollen’s test.
(c)
Interpretation:
Structural formula of the organic product formed when 3-methylpentanal undergoes Benedict’s test has to be drawn.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
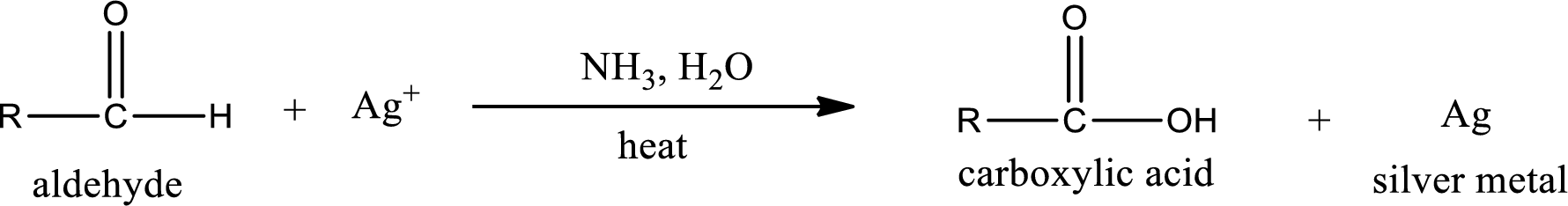
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
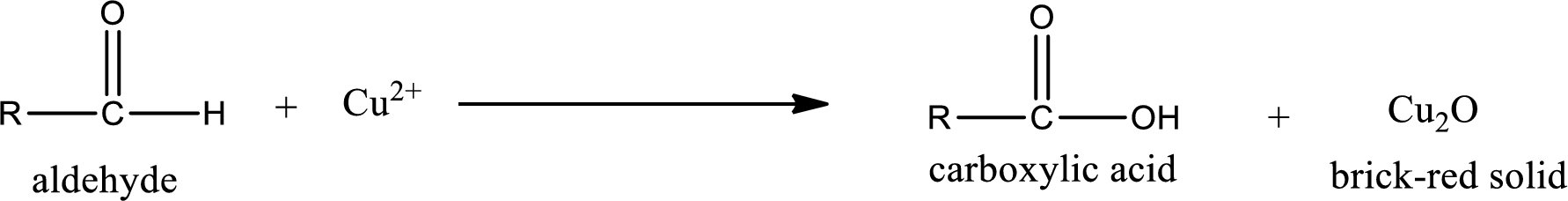
(c)
Answer to Problem 4.77EP
The structure of organic product obtained is,
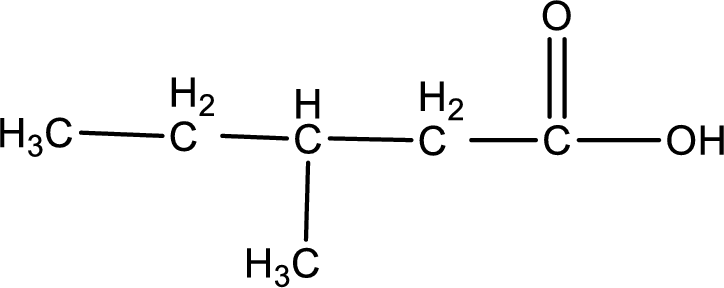
Explanation of Solution
Aldehydes undergo Benedicts’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
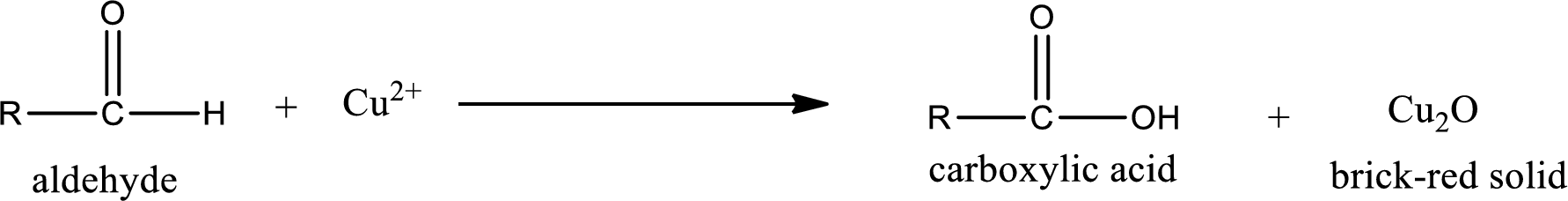
Given aldehyde is 3-methylpentanal and the structure can be given as shown below,
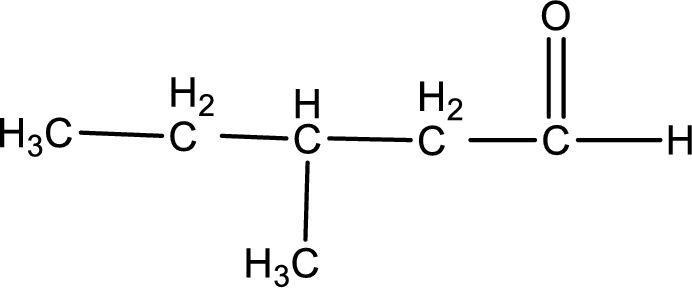
This on reaction with Tollen’s reagent gives carboxylic acid as the product. The structure of the organic product formed and the complete reaction can be given as shown below,
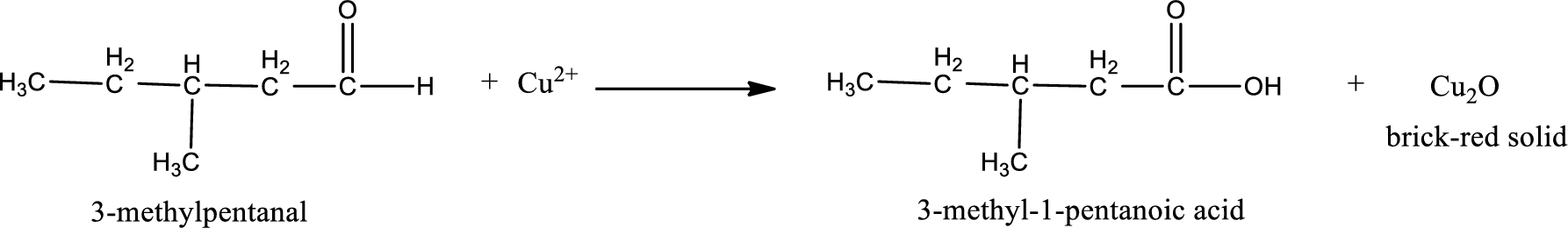
3-methyl-1-pentanoic acid is formed from 3-methylpentanal when it undergoes Benedict’s test.
The structure of the organic product formed is drawn.
(d)
Interpretation:
Structural formula of the organic product formed when 3-pentanone undergoes Benedict’s test has to be drawn.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
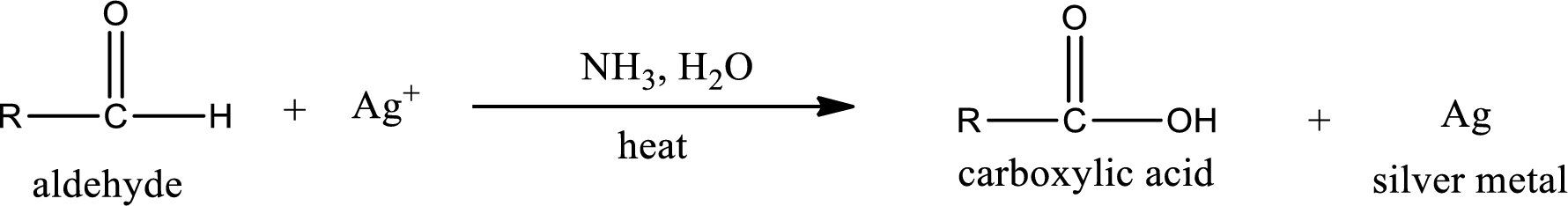
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
(d)
Answer to Problem 4.77EP
3-pentanone does not undergo Benedict’s test.
Explanation of Solution
Aldehydes undergo Benedict’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
Given compound is a ketone. The name of ketone is 3-pentanone and the structure can be given as shown below,
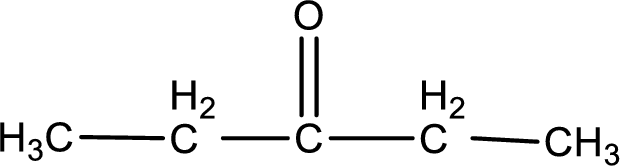
This on reaction with Benedict’s reagent does not give oxidized product. Therefore, no reaction takes place when 3-pentanone undergoes Benedict’s test.
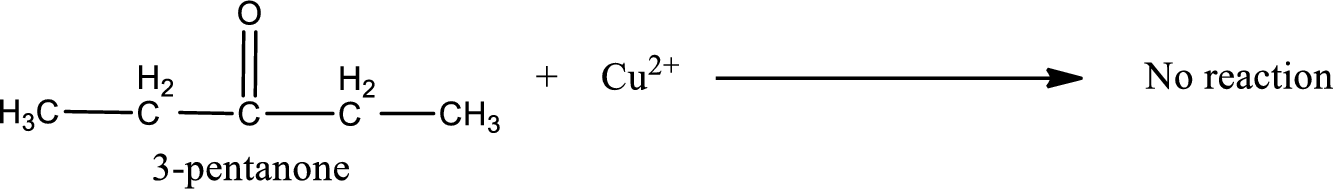
3-pentanone does not undergo Benedict’s test.
No reaction takes place when 3-pentanone undergoes Benedict’s test.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic And Biological Chemistry
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardVanilla flavoring is either extracted from a tropical orchid or synthetically produced from wood pulp by-products. What differences in chemical structure would you expect in these two commercial products: vanilla extract and imitation vanilla extract?arrow_forwardSpilled formaldehyde can be treated with ___ to produce a harmless product that can be easily disposed of. This can be a standard procedure for neutralizing major spills. chlorine bleach glutaraldehyde salt ammoniaarrow_forward
- WHich of the following uses of ethers is not correct a. diethyl ether is used as a propellant for aerosol sprays b. ethers are used as an inert solvent c. solutes for varnishes and lacquers d. ethers used as cooling agentarrow_forwardWhen & how Biological oxidation of an alcohol occurs ?arrow_forwardWhy is acetone a good solvent for many organic compounds?arrow_forward
- Draw the structure of 3-ethylheptanoic acidarrow_forwardwhat is the pentane effect?arrow_forwardEXPLAIN THE INTERACTION BETWEEN THE SOLUTE AND SOLVENT IN TERMS OF THEIR STRUCTURE. 1. Why is Ethanol soluble in water? 2. Why is Acetic Acid soluble in water? 3. WHy is Ester insoluble in water? 4. Why is Ethanal soluble in water? 5. Why is Propanol soluble in water?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





