
Concept explainers
(a)
Interpretation:
The IUPAC name for the ester formed when ethanoic acid and propyl alcohol react has to be assigned.
Concept Introduction:
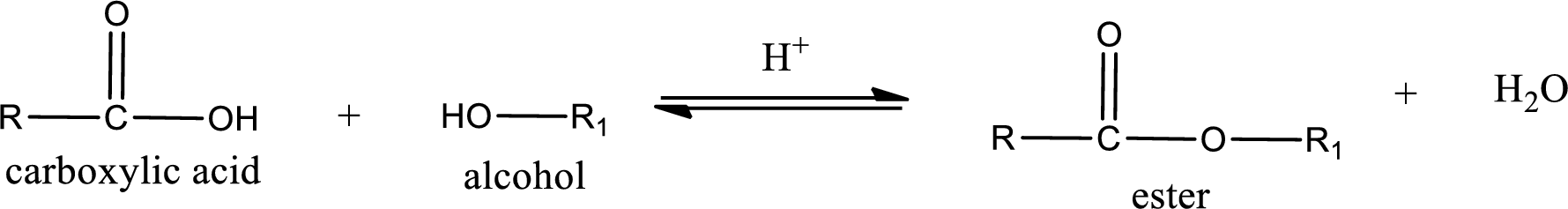
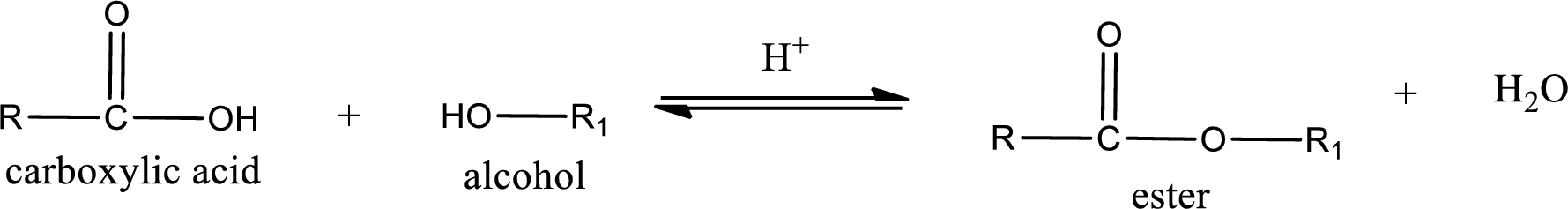
Esters are prepared by condensation of
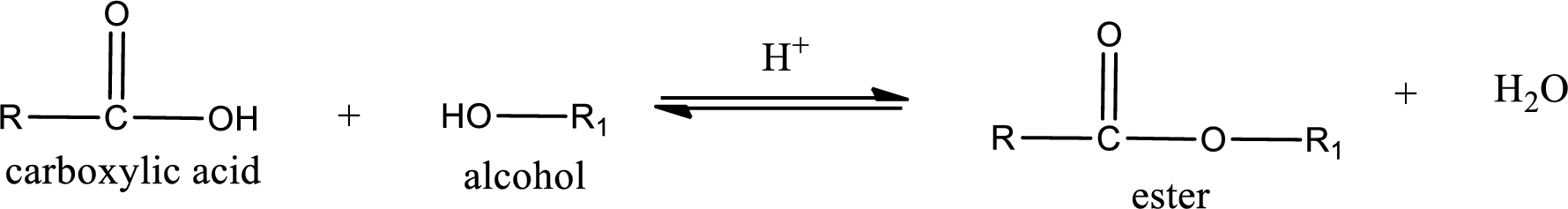
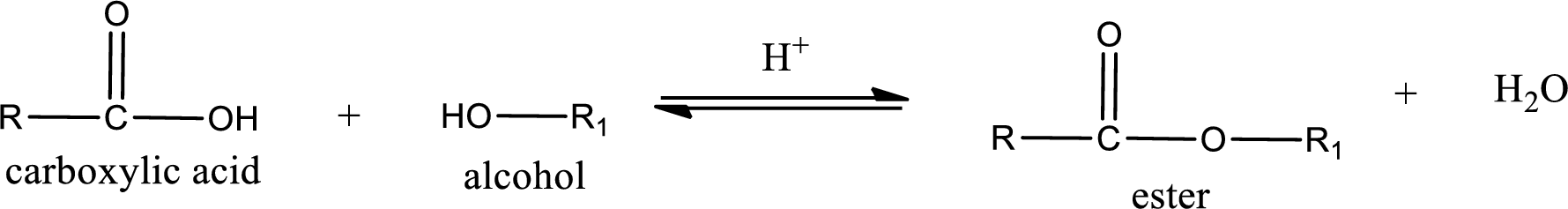
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
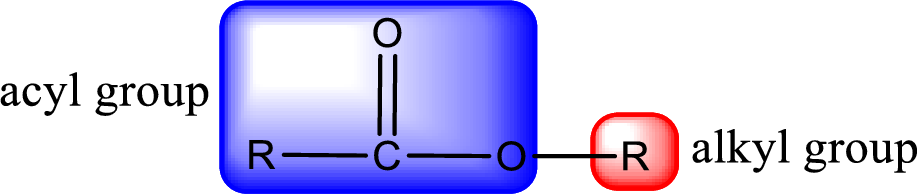
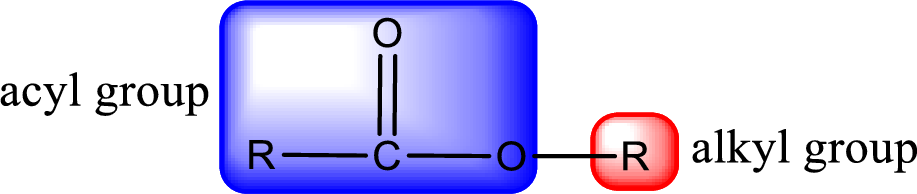
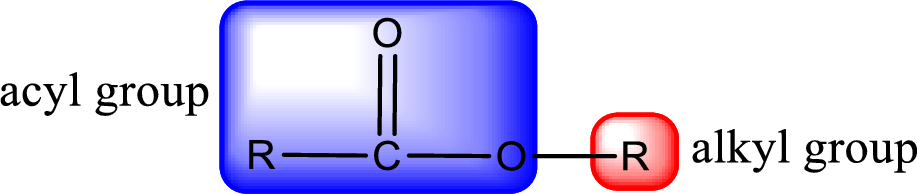
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
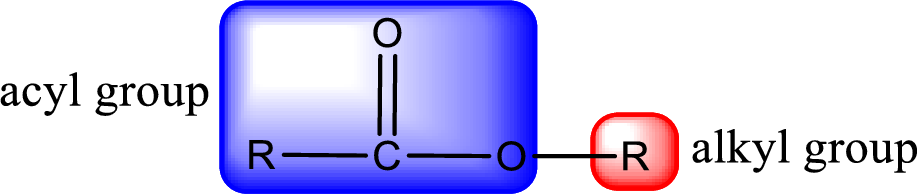
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(a)
Answer to Problem 5.98EP
IUPAC name of the ester formed is propyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,

The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
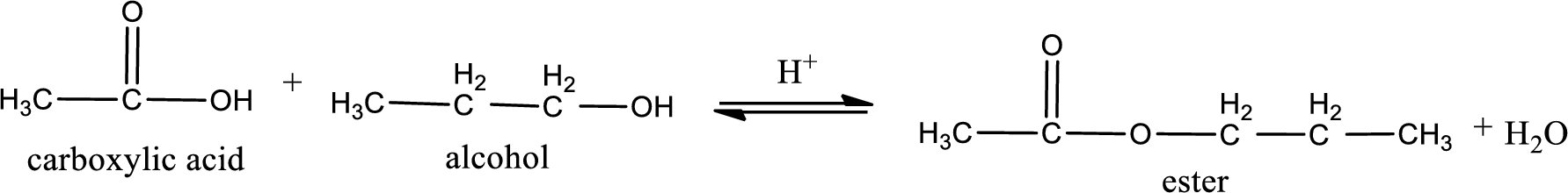
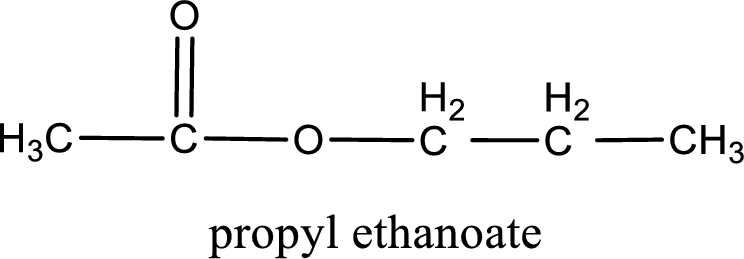
The structure of ester is,
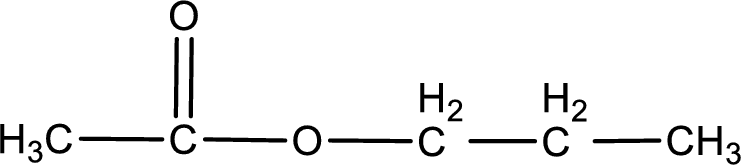
The alkyl and acyl group is identified as shown below,
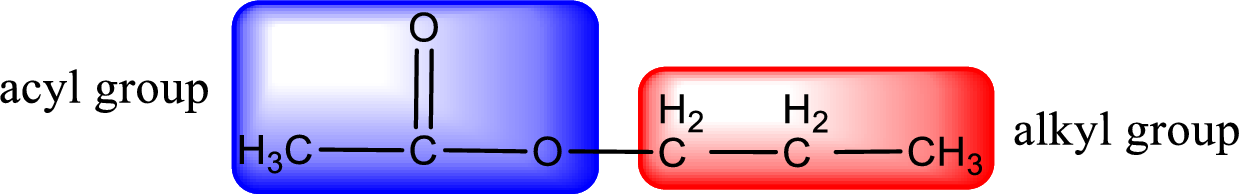
Alkyl group contains three carbon atoms. Hence alkyl group is named as propyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is propyl ethanoate.
IUPAC name of the formed ester is assigned.
(b)
Interpretation:
The IUPAC name for the ester formed when acetic acid and 1-pentanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(b)
Answer to Problem 5.98EP
IUPAC name of the ester formed is pentyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
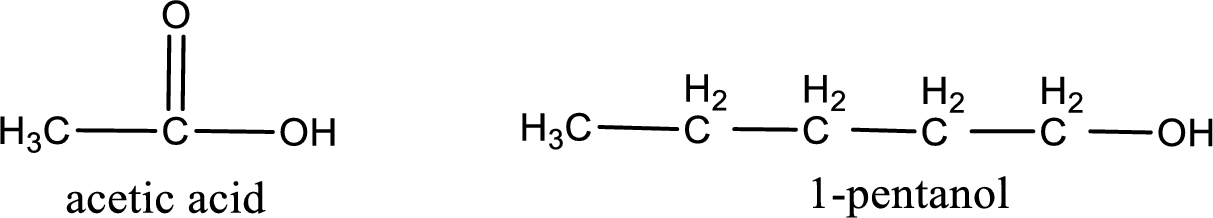
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
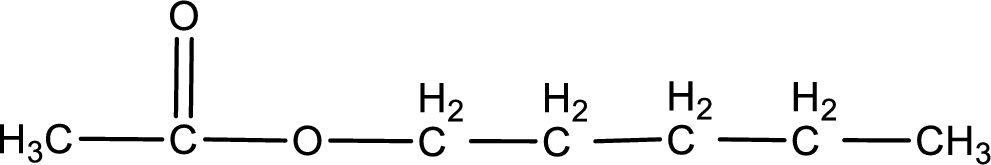
The alkyl and acyl group is identified as shown below,
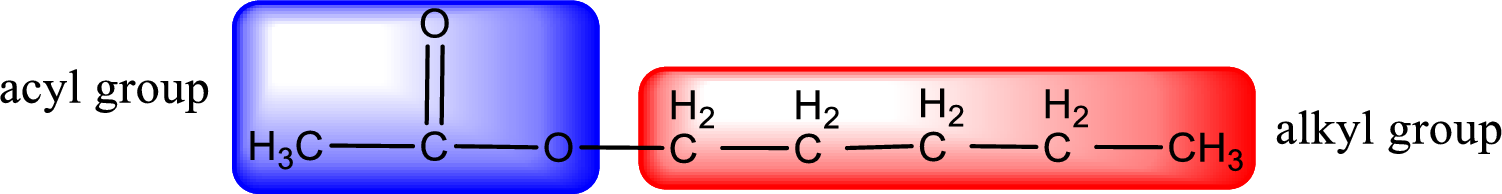
Alkyl group contains five carbon atoms. Hence alkyl group is named as pentyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is pentyl ethanoate.
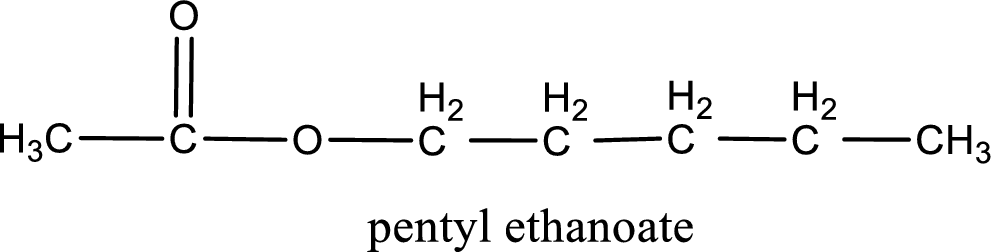
IUPAC name of the formed ester is assigned.
(c)
Interpretation:
The IUPAC name for the ester formed when acetic acid and 2-pentanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(c)
Answer to Problem 5.98EP
IUPAC name of the ester formed is 1-methylbutyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
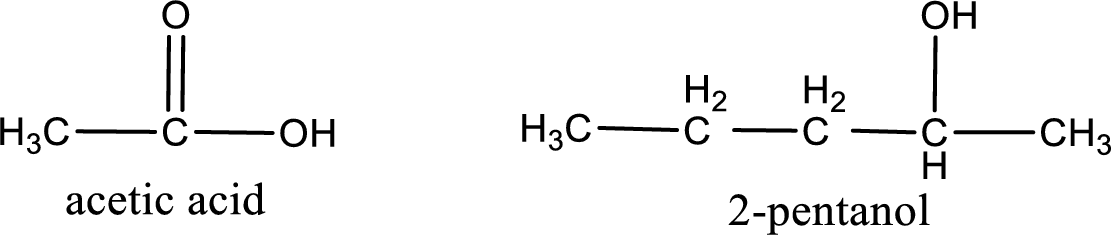
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
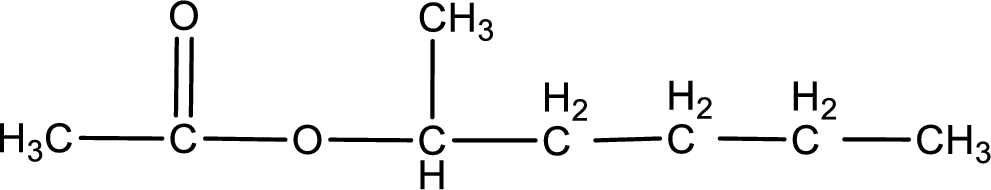
The alkyl and acyl group is identified as shown below,
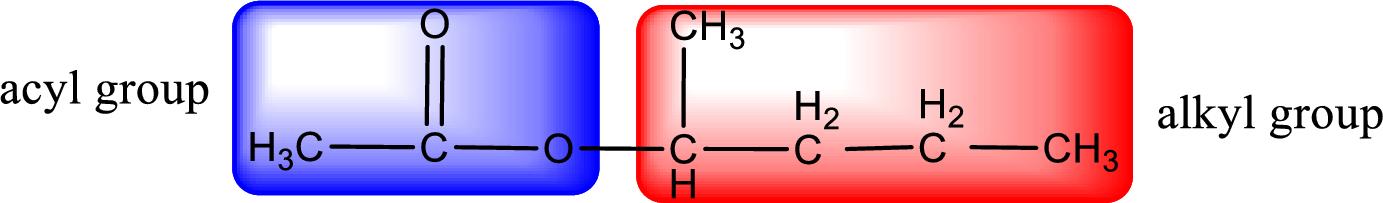
Alkyl group contains five carbon atoms. Four carbon in a long chain with a methyl group substituted in the first carbon atom. Hence alkyl group is named as 1-methylbutyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is 1-methylbutyl ethanoate.
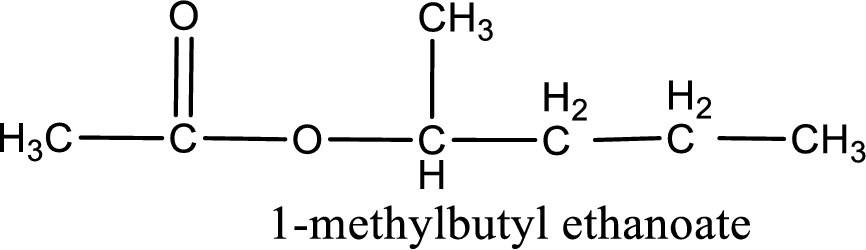
IUPAC name of the formed ester is assigned.
(d)
Interpretation:
The IUPAC name for the ester formed when ethanol and benzoic acid react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(d)
Answer to Problem 5.98EP
IUPAC name of the ester formed is ethyl benzoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
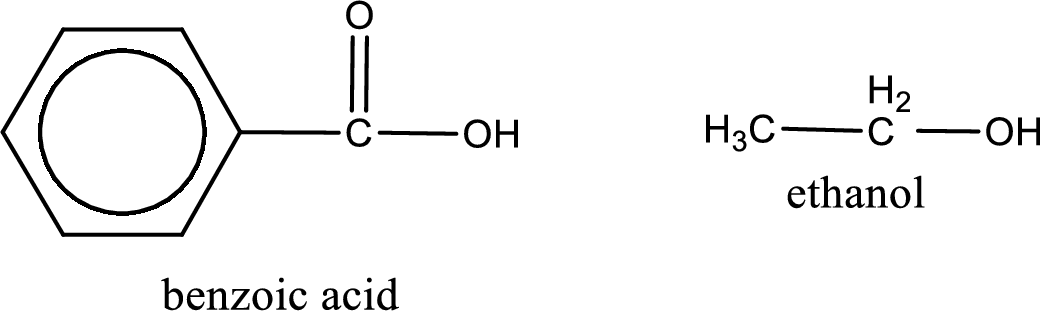
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
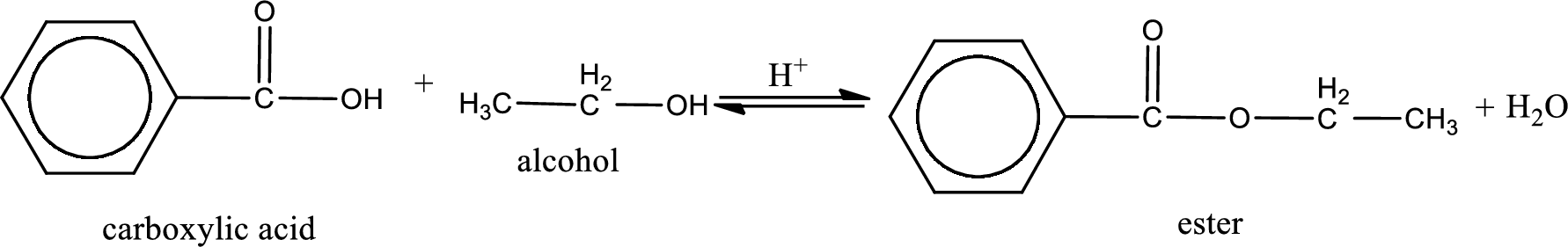
The structure of ester is,
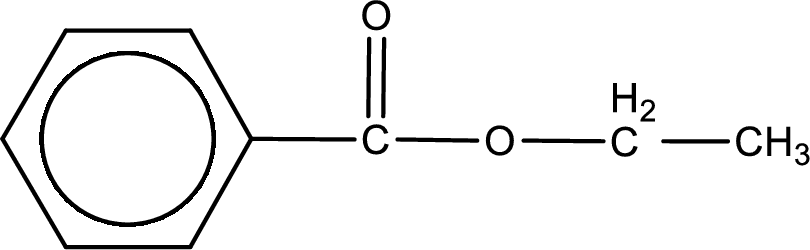
The alkyl and acyl group is identified as shown below,
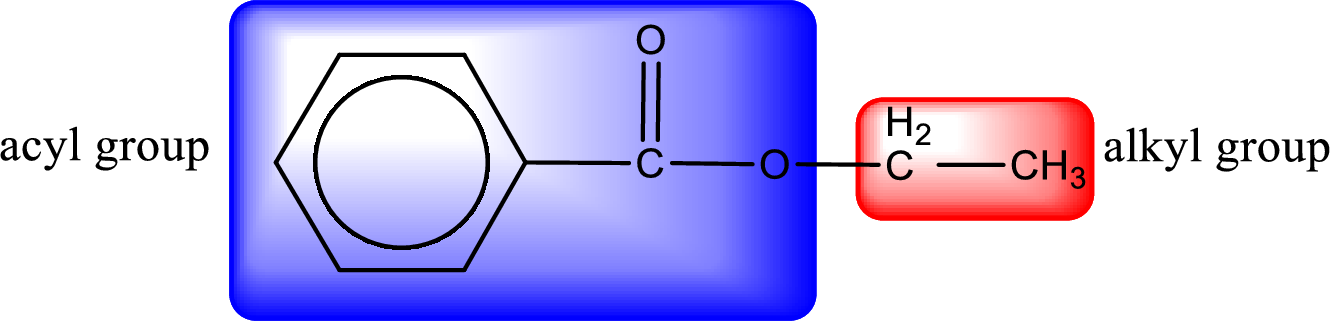
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains a benzene ring structure. The IUPAC name of carboxylic acid that contains benzene ring is benzoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as benzoate. Therefore IUPAC name of the given ester is ethyl benzoate.
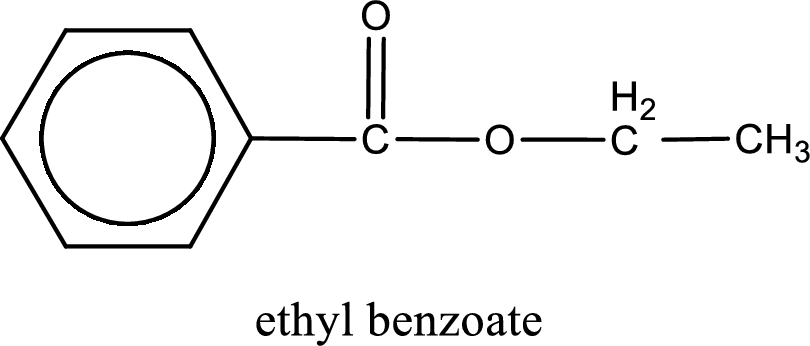
IUPAC name of the formed ester is assigned.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic And Biological Chemistry
- The most typical reaction involving unsaturated hydrocarbons is: a) Replacement b) oxidation c) additions d) hydrolysisarrow_forwardDefine the structure of a carboxylic acid ?arrow_forward8. The organic starting materials for the preparation of an ester could be_________A. a ketone and alcohol B. water and oxygen C. an acid and alcohol D. alkane and aldehydearrow_forward
- What reagents are used in the esterification of Alcohols and Phenols? a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloridearrow_forwardWrite the complete condensed reaction and write the name of the final product. A. oxidation of 2-methyl-3-pentanol B. Propanoic acid plus methanamine C. Benzoic acid+propanolarrow_forwardDifferentiate the two hydrolytic reactions of estersarrow_forward
- What are the different carboxylic acid derivatives? Describe each. What is the specific characteristics of esters and their importance? Enumerate the importance of esterification process in pharmacy and in medicine.arrow_forwardGive the structural formulas for:(i) Methyl Ethanoate(ii) Ethyl ethanoateWrite two uses of Ester?arrow_forwardSelect the general class of compounds you would expect to have the highest boiling point. A. Aldehydes B. Esters C. Alkanes D. Alcoholsarrow_forward
- In organic chemistry, oxidation reactions A transform aldehydes into ketones B decrease the oxidation number of the elements involved C increase the number of carbon-oxygen bonds D) convert ketones into carboxylic acids E produce aldehydes from secondary alcoholsarrow_forwardWhat is the functional isomer of butanal? butanoic acid 1-butanol butanone methyl propanoate Other:arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





