
Concept explainers
(a)
Interpretation: The highest-energy species in the reactants and the highest-energy species in the products should be circled and explanation about reason behind no uphill curve even when generation of oxygen with a uni-negative charge occurs in below reaction should be given.
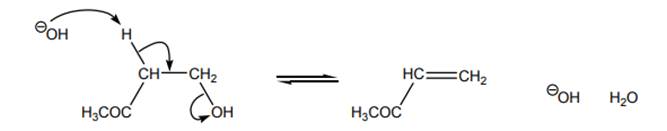
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along y-axis and reaction coordinate along x axis. Reaction proceeds when the reactant molecules collide and have proper orientation. The pathway from reactant to product involves transition state. A transition state is a hypothetical state that gives clear picture of the orientation of reactant molecules during collision that is a process of the bonds formed and broken during the reaction. Transition state is the highest energy state of a reaction.
The intermediate involved usually have higher potential energy than reactants species as they are formed transiently and fall back to form products that are more stable and hence reduced in potential energy.
(d)
Interpretation: Energy diagram that best describes the below reaction should be identified.
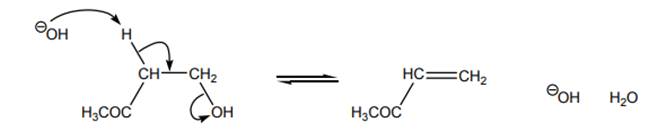
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along y-axis and reaction coordinate along x axis. Reaction proceeds when the reactant molecules collide and have proper orientation. The pathway from reactant to product involves transition state. A transition state is a hypothetical state that gives clear picture of the orientation of reactant molecules during collision that is a process of the bonds formed and broken during the reaction. Transition state is the highest energy state of a reaction.
The intermediate involved usually have higher potential energy than reactants species as they are formed transiently and fall back to form products that are more stable and hence reduced in potential energy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
