
Concept explainers
Interpretation: The most likely hydride shift that will occur for each of the below carbocations should be depicted with curved arrow and reason behind lowered potential energy for thus newly formed carbocation should be explained.
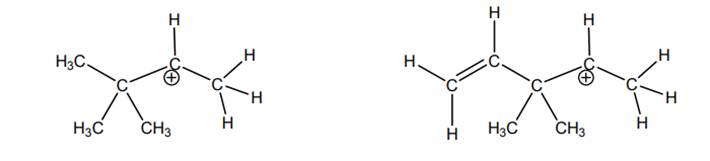
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
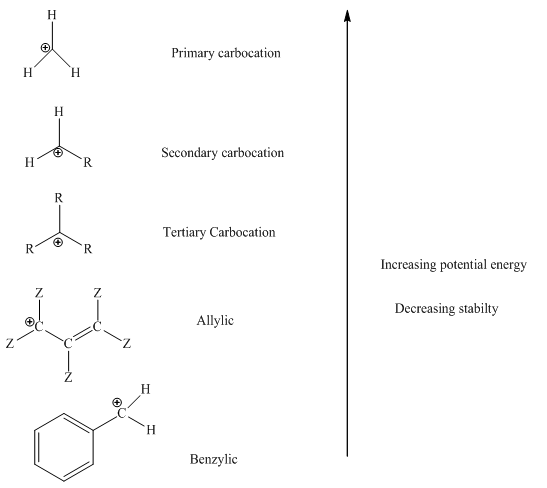
The order of relative stability of various possible carbocation species is as follows:
Whenever possibility to attain lower energy by rearrangement is there then hydride or alkyl shift may occur and results in more stable carbocation. This type of rearrangement is highly favorable in polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- Answer with proper explanation, I will give you an upvote!!arrow_forwardNot sure how to correctly move the carbocation so that it is at its most stable, tertiary , in the correct order of steps and correct placement of the pi bondarrow_forwardCan you show the mechenismim for both the major and minor product?arrow_forward
- Please help with this question, the full step by step answer would be greatly appreciated, I will upvote!!arrow_forwardFor this question, would both of these products be anti-Markovnikov products? I'm confused why the Markovnikov product with a 1,2-hydride shift wouldn't be displayed here (or for the purposes of the question, it may have been purposely left out)?arrow_forwardConsider the following carbocations. Circle all carbocations that would rearrange.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
