
Concept explainers
Interpretation: Reason for rearrangement shown below shows downhill in terms of potential energy should be explained.
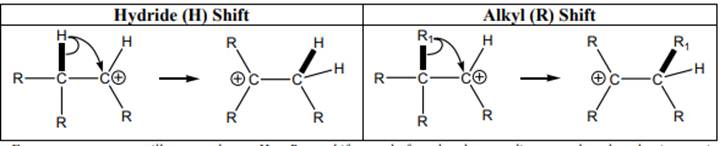
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
The carbocation formed may rearrange itself to a more stable carbocation through a
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Custom eBook for Organic Chemistry
- pts) Show any reaction that would involve the rearrangement of an intermediate carbocation.arrow_forwardFollow the curved arrows and draw the product of this reaction. Ph • You do not have to consider stereochemistry.arrow_forward함 H Follow the curved arrows and draw the product of this reaction. . You do not have to consider stereochemistry.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Probler Drawing Arrows THF Na :Cl:O 'N. NaO C:O Drag Toarrow_forwardBrarrow_forwardThis content is protected and may nag shared t 4. Consider the following reaction. This reaction is a multi-step reaction. [1] Draw the mechanism for each step of this reaction using curved arrow notation, include all lone pairs of electrons if they are directly involved in the reaction. [2] Identify the arrow pushing pattern (mechanistic pattern: nucleophilic attack, loss of leaving group, or proton transfer) for each step. [3] Draw a transition state for each step. H-OSO3H CH3 00 H H3C-O-H Хосно H3C-O-H Harrow_forward
- draw out mechanism (either E1 or E2) for this combined with CH3OH (arrows, intermediates, etc should be shown). Circle the less prevalent and more prevalent productsarrow_forwardDraw the appropriate Newman projection which leads to the major E2 ELIMINATION product. Hint: Review Chapter 10.3, the elimination can only occur if the leaving group (Cl, Br, I, OTs, etc) and beta-hydrogen atom (H) are in anti-periplanar conformation. Red bond = the location of a double bond in the final product and the direction of a Newman projection: draw anti-periplanar conformation (leading to major elimination product) A B C D CH3 CI CH3 Et CH3 H CH3 CH3 H H H H3C H3C H3C H3C H Et CI H Et CI CI Et Et Et Et H Newman Newman Newman Newmanarrow_forwardConsider the reaction between 2-methyl-2-butanol and HBr, shown below. но HBr Brarrow_forward
- 7. Draw the complete, detailed mechanism (curved arrows) for each of the following reactions occurring under SN1 conditions and under SN2 conditions. Pay attention to the stereochemistry of the product. al + SN1 mechanism SN2 mechanism Br SN1 mechanism + KBr SN2 mechanism NaOH CI + NaOCH3 SN1 mechanism SN2 mechanismarrow_forwardgiven reaction (see image), draw the curly arrows for the mechanism to show the movement of electron pairsarrow_forwardDraw the missing curved arrow notation for the rearrangement below. v 1st attempt O Add the missing curved arrow notation.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
