
Concept explainers
Interpretation: The most likely hydride shift that will occur for each of the below carbocations should be depicted with curved arrow and reason behind lowered potential energy for thus newly formed carbocation should be explained.
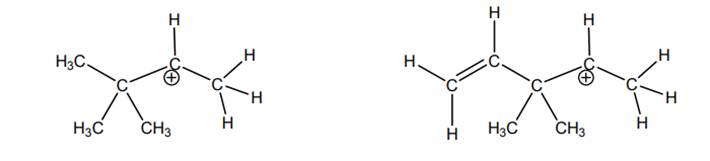
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
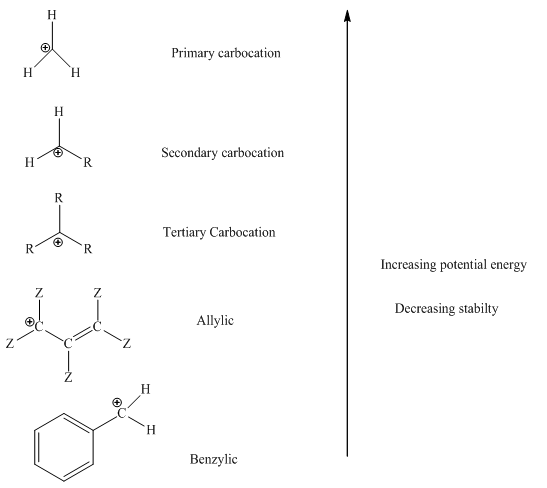
The order of relative stability of various possible carbocation species is as follows:
Whenever possibility to attain lower energy by rearrangement is there then hydride or alkyl shift may occur and results in more stable carbocation. This type of rearrangement is highly favorable in polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Custom eBook for Organic Chemistry
- I am supposed to draw the molecule with the carbocation rearranged to where it is more stable. I was going to to a hydride shift and move it to the carbon that is highlighted in red. The red carbon means it wont let me place it there for some reason. Is that not the most stable position to put it in or is there a better spot?arrow_forwardWhy does Hammett Equation only apply to meta and para substituted rings and not others? Explainarrow_forwardPredict the product(s) OR starting material of the following reactions. Remember hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put only the organic molecules in the boxes.arrow_forward
- Consider the following carbocations. Circle all carbocations that would rearrange.arrow_forwardDraw a curved arrow mechanism for the substitution reaction that will occur with the alkyl halide and nucleophile below, adding steps as necessary. Be sure to include all nonbonding electrons and all nonzero formal charges, and show all species that react or are formed in this reaction.arrow_forwardPredict the product or starting material of the following alkene addition reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist! Put your answers (organic products only) in the indicated boxes.arrow_forward
- CO2 Follow the curved arrows and draw the product of this reaction. You do not have to consider stereochemistry.arrow_forwardPredict the products or starting material of the following alkene addition reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist! Put your answers (organic products only) in the indicated boxes.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

