
Interpretation: The most likely hydride shift that will occur for each of the below carbocations should be depicted with curved arrow and reason behind lowered potential energy for thus newly formed carbocation should be explained.
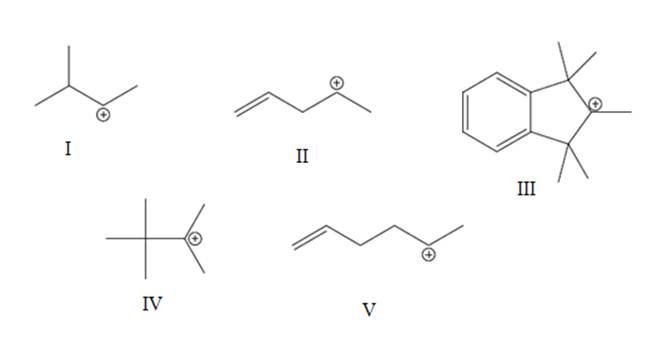
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
The order of relative stability of various possible carbocation species is as follows:
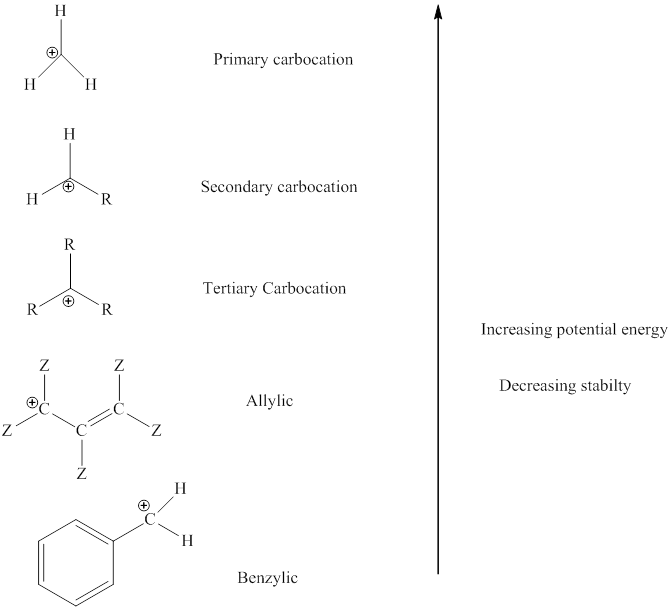
Thus, whenever possibility to attain lower energy by rearrangement is there hydride or alkyl shift may occurs and results in more stable carbocation. This type of rearrangement is highly favorable in polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Custom eBook for Organic Chemistry
- Using arrows, show the electron rearrangement that takes place in the reaction.arrow_forward149) Why is a tertiary alcohol more reactive with hydrogen halides, than secondary alcohols? Because secondary alcohols are smaller and therefore less reactive for the attack of halide nucleophile. Because tertiary alcohols have more hydrogen atoms available for substitution reactions. Because a tertiary alcohol can form a more stable carbocation as an intermediate in the reaction. Only the 1st and the 2nd statements are acceptable.arrow_forwardWhy does Hammett Equation only apply to meta and para substituted rings and not others? Explainarrow_forward
- As we will learn, many antioxidants–compounds that prevent unwanted radical oxidation reactions from occurring–are phenols, compounds that contain an OH group bonded directly to a benzene ring.a.) Explain why homolysis of the O–H bond in phenol requiresconsiderably less energy than homolysis of the O–H bond in ethanol(362 kJ/mol vs. 438 kJ/mol).b.) Why is the C–O bond in phenol shorter than the C–O bond in ethanol?arrow_forwardWhen piperidine undergoes the series of reactions shown here, 1,4-pentadiene is obtained as the product. When the four different methyl- substituted piperidines undergo the same series of reactions, each forms a different diene: 1,5-hexadiene; 1,4-pentadiene; 2-methyl-1,4-pentadiene; and 3-methyl-1,4-pentadiene. Which methyl-substituted piperidine forms which diene?arrow_forwardBoth questions related to each other. Need solution for both with explanationarrow_forward
- Please help me with the organic chemistry problem below: Consider the reaction below: (Check the attached image) (it is between Furan and maleic anhydride, a DIels-Alder reaction) a) Will this reaction for an endo product (with a melting point of 80-81 degrees) or the exo product (with a melitng point of 114 degrees)? b) Carefully explain why the product must have been formed the way it did (exo or endo). c) Provide a mechanism for this reaction.arrow_forwardNeed help solving this, please show your work with the answer!arrow_forward6.The three compounds below can form a carbocation under aqueous acidic conditions. Draw the structure of Carbocation. || =arrow_forward
- Please don't provide handwritten solution ....arrow_forwardThe following reaction conditions gives a mixture of two addition products A and B shown below. CI. HCI Ph Ph Ph Ph Ph A B via rearrangement via rearrangement >Draw< a clear mechanism using electron-flow arrows showing the formation of both products (A and B) from the starting materials. Transition states are not required and ignore the stereochemistry in this question. Do not use any other compounds in your answer except those given. Note that this is not a synthesis question. OYork rk.Unesk 020arrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. + HCI You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right Separate resonance structures using the symbol from the drop-down menu. ← - CHA ? n ChemDoodlearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



