Table 1: Volumes in of iodine in mL used to titrate mixtures of 10 mL of reaction mix and 10 mL of urine. student trial 1 trial 2 trial 3 total volume (mL) a 12.6 11.8 12.5 10.6 300 10.6 10.7 290 11.1 11.0 12.7 10.9 405 13.0 12.5 349 F mg vit. C mL iodine TV (mL/hour) 10 mL urine aliquot TVC (mg/hour) =z (mL) · CF %3D What is the hourly vitamin C excretion rate (mg/h) of student b if the CF is 1.06? (include no more than two decimals in your answer, e.g.: 123.45, and do not type the units)
Table 1: Volumes in of iodine in mL used to titrate mixtures of 10 mL of reaction mix and 10 mL of urine. student trial 1 trial 2 trial 3 total volume (mL) a 12.6 11.8 12.5 10.6 300 10.6 10.7 290 11.1 11.0 12.7 10.9 405 13.0 12.5 349 F mg vit. C mL iodine TV (mL/hour) 10 mL urine aliquot TVC (mg/hour) =z (mL) · CF %3D What is the hourly vitamin C excretion rate (mg/h) of student b if the CF is 1.06? (include no more than two decimals in your answer, e.g.: 123.45, and do not type the units)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
100%
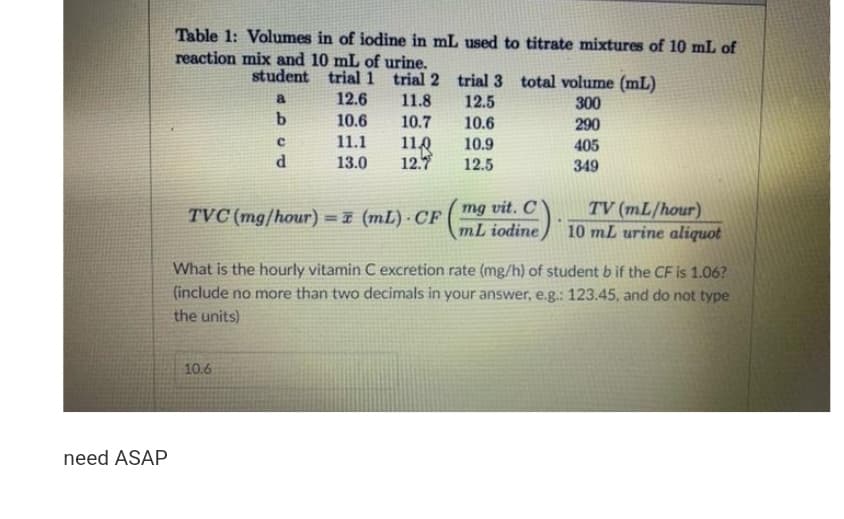
Transcribed Image Text:Table 1: Volumes in of iodine in mL used to titrate mixtures of 10 mL of
reaction mix and 10 mL of urine.
student trial 1 trial 2 trial 3 total volume (mL)
a
12.6
11.8
12.5
300
290
b.
10.6
10.7
10.6
11.1
118
12.
10.9
405
13.0
12.5
349
mg vit. C
mL iodine
TV (mL/hour)
10 mL urine aliquot
TVC (mg/hour) =7 (mL) · CF
!!
What is the hourly vitamin C excretion rate (mg/h) of student b if the CF is 1.06?
(include no more than two decimals in your answer, e.g.: 123.45, and do not type
the units)
10.6
need ASAP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What is the highest hourly vitamin C excretion rate (mg/h) of these four students if the CF is 1.06? (include no more than two decimals in your answer, e.g.: 123.45, and do not type the units)
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning