
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.45SP
A student found an old bottle labeled “thymol” on the stockroom shelf. After noticing a pleasant odor, she obtained the following mass, IR, and NMR spectra. The NMR peak at δ4.8 disappears on shaking with D2O. Propose a structure for thymol, and show how your structure is consistent with the spectra. Propose a fragmentation to explain the MS peak at m/z 135, and show why the resulting ion is relatively stable.
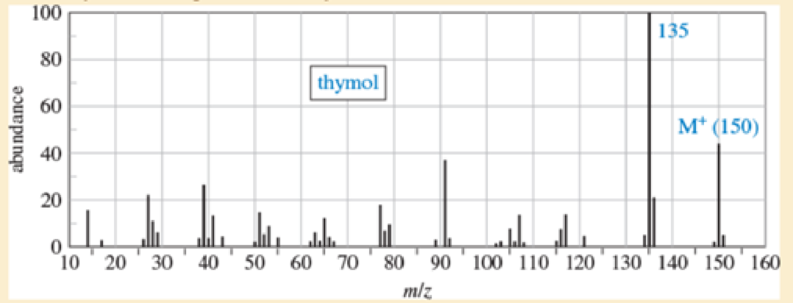
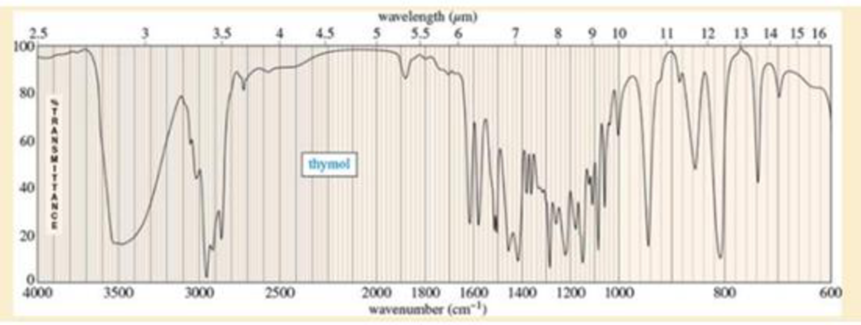
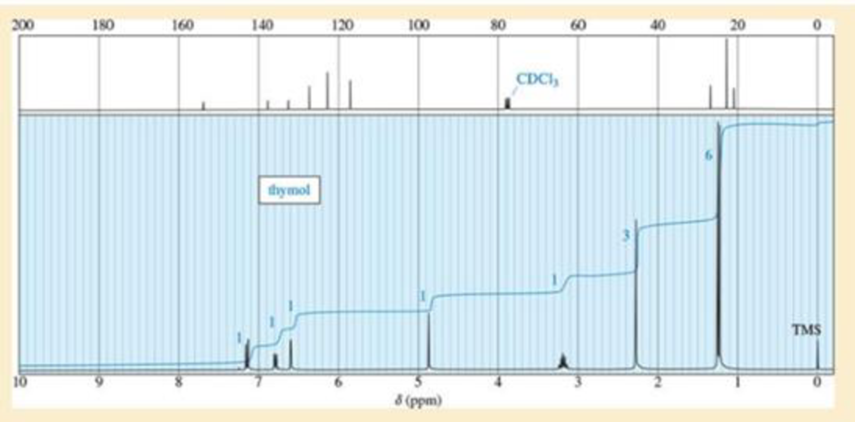
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The H NMR spectra corresponds to a hydrocarbon with the molecular formula C6H₁4. Deduce the structure from
the spectra.
Draw the unknown compound.
'H NMR
12 H
Select
Draw
Rings
More
1.5 1.4
2 H
1.5 1.0 0.5 0.0
300-MHz H NMR
spectrum ppm (8)
0.9 0.8
(CH3)4Si
G
G
Q2E
An unknown organic compound is isolated. The compound produces a molecular ion at m/z = 101. There is no significant M+2 peak. The IR spectrum shows peaks ~2950 cm'. It does not have any peaks
above 3000, nor around 1600-1800 or 1050 cm1. The 'H-NMR and 13C-NMR spectra are shown below. What is the name of this compound (IUPAC and common name are both acceptable)?
3H
2H
PPM
20
10
30
PPM
50
40
The 'H NMR and 13C spectra of a compound with a molecular formula of C6H12O2 are shown below.
1. Name the compound in the textbox below.
2. Draw a possible structure for this compound.
1Η NMR
2H 2H
2H
3HPPM
3H
13C NMR
220
200
100
140
100
120
PPM
Chapter 16 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How could you use 1H NMR, 13C NMR, and IR spectroscopy to help you distinguish between the following structures?arrow_forwardCompound A, C8H10, yields three substitution products, C8H9Br, on reaction with Br2. Propose two possible structures for A. The 1H NMR spectrum of A shows a complex four-proton multiplet at 7.0 δ and a sixproton singlet at 2.30 δ. What is the structure of A?arrow_forwardTreatment of 3,4-dibromohexane with strong base leads to loss of 2 equivalents of HBr and formation of a product with formula C6H10. Three products are possible. Name each of the three, and tell how you would use 1H and 13CNMR spectroscopy to help identify them. How would you use UV spectroscopy?arrow_forward
- What is the structure of an unknown compound with molecular formula C6H15N that gives the following 1H NMR absorptions: 0.9 (singlet, 1 H), 1.10 (triplet, 3 H), 1.15 (singlet, 9 H), and 2.6 (quartet, 2 H) ppm?arrow_forwardMatch the spectra to the structures: 3-bromotoluene and methyl 4-methoxybenzoate. CH 3 3-bromotoluene 200 Spectrum B 200 Br (ignore the CDCI3 signal) CH3O methyl 4-methoxybenzoate OCH3 Spectrum A 100 100arrow_forwardChoose the structure corresponding to the given 1H and 13C NMR spectraarrow_forward
- Draw the structure of the compound identified by the simulated 'H NMR and ¹3C NMR spectra. The molecular formula of the compound is C₁0H₁2O. (Blue numbers next to the lines in the 'H NMR spectra indicate the integration values.) ¹H NMR 1H 2H 2H 2H 2H 3H IT 111 10 8 6 8 (ppm) 13C NMR III. 220 200 180 160 140 60 40 20 100 8 (ppm) Deduce the structure from the spectra. Select Draw More CHO 2 Ć Rings 120 80 Erase Q 2 Qarrow_forwardDraw the structure of the compound identified by the simulated 'H NMR and 13C NMR spectra. The molecular formula of the compound is C1,H120. (Blue numbers next to the lines in the 'H NMR spectra indicate the integration values.) Η NMR 1H 2H 2H 2H 2H 3H| 10 8 4 2 8 (ppm) 13C NMR 220 200 180 160 140 120 100 80 60 40 20 d (ppm)arrow_forwardWhat is the structure of the compound if the mass spectrum shows M+ = 72, IR shows peak near 1720 cm-1, 13C-NMR shows 4 lines. The proton NMR is: 2.4 ppm (2H quartet) 2.1 ppm (3H singlet) 1.1 ppm (3H triplet)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY