
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.49SP
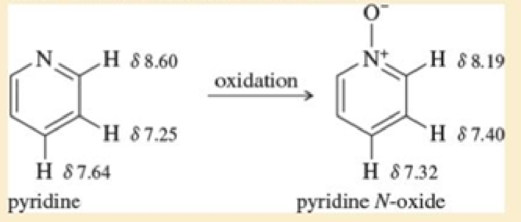
The proton NMR chemical shifts of the hydrogens in pyridine are shown. These are typical
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rank the following compounds in order of increasing acidity (1 = least acidic, 3 = most acidic) and in the space provided use resonance (of the conjugate base) to explain why the compound you have labelled “3” is the most acidic.
Vanillic acid is the oxidized phenolic derivative of vanillin. By using Proton
Nuclear Magnetic Resonance ('H-NMR) spectroscopic technique, point out
the difference that can be observed between vanillin and vanillic acid in
terms of chemical shift based on its chemical structures.
но
Он
Vanillic acid
Vanillin
The 1H- and 13C-NMR data of an ester of molecular formula C6H10O2 are given below. Also shown are the COSY and HETCOR NMR spectra of the ester. Draw the structure of the ester, explaining how you reach your conclusion.
1H-NMR: 7.20-6.90 (1H), 5.85 (1H), 4.16 (2H), 1.88 (3H), 1.31 (3H) ppm
13C-NMR: 166.7, 144.5, 123.0 , 60.2, 18.0, 14.3 ppm
Chapter 16 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1, 6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at -0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.arrow_forwardCompounds A and B are isomers having the molecular formula C4H8O3. Identify A and B on the basis of their 1H NMR spectra.Compound A: δ 1.3 (3H, triplet); 3.6 (2H, quartet); 4.1 (2H, singlet); 11.1 (1H, broad singlet)Compound B: δ 2.6 (2H, triplet); 3.4 (3H, singlet); 3.7 (2H triplet); 11.3 (1H, broad singlet)arrow_forwardAcridine is a heterocyclic aromatic compound obtained from coal tar that is used in the synthesis of dyes. The molecular formula of acridine is C13H9N, and its ring system is analogous to that of anthracene except that one CH group has been replaced by N. The two most stable resonance structures of acridine are equivalent to each other, and both contain a pyridine-like structural unit. Write a structural formula for acridine.arrow_forward
- Deduce the identity of the following compound from the spectral data given. C8H10: 1H NMR, 6 1.20 (3H, triplet), 2.60 (2H, quartet), 7.12 (5H, singlet) (ppm); IR, 3050, 2970, 1600 cm-1; MS, m/z 91arrow_forwardPhenol (hydroxybenzene) behaves as a weak acid. a) Write out the equilibrium equation for its partial dissociation in water. b) Write out the expression for the acid dissociation constant, Ka. d) Draw the conjugate base of phenol and show how it is stabilised by resonance. e) Compare and explain the acidity of phenol (p = 9.9) with that of: cyclohexanol (pk = 16.0) 3-fluorophenol (pK₁ = 9.3) 4-acetylphenol (pK, = 8.1)arrow_forwardKeeping with the theme of autumn, one of Dr. Danahy’s favorite molecules is caffeic acid due to its presence in pumpkins. This structure serves as an antioxidant and is one of many found within pumpkins. Despite its name, it bears no resemblance to caffeine. Answer the following questions about caffeic acid. The carbonyl stretch for caffeic acid is unusually low for a carboxylic acid at 1646 cm-1. For reference, the carbonyl stretch for propanoic acid is 1716 cm-1. Explain why the carbonyl stretch occurs at a lower wavenumber for caffeic acid.arrow_forward
- describe the NMR spectra pattern of benzoic acidarrow_forwardThe NMR spectra are shown in parts c, d for isomeric compounds with formula C10H12O2. Their infrared spectra show strong bands near 1735 cm-1 . Make noattempt to interpret the aromatic proton area between 7.0 and 7.5 ppm except todetermine the number of protons attached to the aromatic ring. Draw the structures of the compounds.arrow_forwardBased on the structures given below, which of the following statements is entirely true? Quinoline NH Isoquinoline Indole Isoindole All four compounds are non-aromatic, with 12 pi-electrons each and are equal in basicity. Only quinoline and Isoquinoline having 10 pi-electrons each are aromatic while indole and isoindole are antiaromatic having 8 pi-electrons each The nitrogen atoms of quinoline and isoquinoline are sp2 hybridized while the nitrogen atoms of indole and isoindole are sp³ hybridized. All four compounds are aromatic, with 10 pi-electrons each and are equal in basicity. All four compounds are aromatic, with 10 pi-electrons each, and with quinoline and isoquinoline being stronger bases than indole and isoindarrow_forward
- Based on the structures given below, which of the following statements is entirely true? N Quinoline NH Isoquinoline Indole Isoindole All four compounds are non-aromatic, with 12 pi-electrons each and are equal in basicity. All four compounds are aromatic, with 10 pi-electrons each and are equal in basicity. Only quinoline and isoquinoline having 10 pi-electrons each are aromatic while indole and isoindole are antiaromatic having 8 pi-electrons each. All four compounds are aromatic, with 10 pi-electrons each, and with quinoline and isoquinoline being stronger bases than indole and isoindole. The nitrogen atoms of quinoline and isoquinoline are sp2 hybridized while the nitrogen atoms of indole and isoindole are sp³ hybridized Previousarrow_forwardCiprofloxacin is a member of the fluoroquinolone class of antibiotics.(a) Which of its rings are aromatic?(b) Which nitrogen atoms are basic?(c) Which protons would you expect to appear between d 6 and d 8 in the proton NMR spectrum?arrow_forwardCyclopropenones are described as having aromatic character. How would you account for this, given that the ring contains three π-electrons?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY