
Interpretation:
The condensed structural formula for four different isomers of molecular formula,
Concept introduction:
The molecules having same molecular formula but structural arrangement of atoms in the molecule is different are said to be isomers of each other.
Answer to Problem 71A
Isomer I:

Isomer II:

Isomer III:
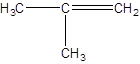
Isomer IV:
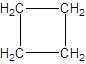
Explanation of Solution
The given molecular formula is
- All four-carbon atoms are written in a straight chain and double bond at first position.
- All four-carbon atoms are written in a straight chain and double bond at second position.
- Making a three-carbon atoms straight chain with a double bond at position 1 and a methyl substituent at second carbon atom:
- Making a cycloalkane of four carbon atoms bonded by single bonds only:
Isomer I:
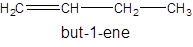
Isomer II:
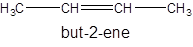
Isomer III:
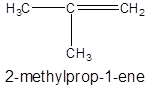
Isomer IV:
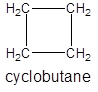
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (14th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Organic Chemistry (9th Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





